Quorum Sensing and Biofilm Formation: The Impact on Food Safety and Quality
Quorum sensing and biofilm formation play a critical role in microbial contamination within food and dairy processing environments. Quorum sensing (QS), a bacterial communication system, is at the heart of biofilm formation, enabling bacteria within the biofilm to coordinate their actions and enhance their resistance to environmental threats. With the aid of quorum sensing, bacteria within a biofilm become encased in a protective extracellular matrix, allowing them to adhere firmly to surfaces and resist traditional cleaning methods, creating persistent reservoirs of pathogens and spoilage organisms that can contaminate food and dairy products. Once biofilms form on surfaces, they become extremely difficult to remove, which poses significant challenges to maintaining hygiene and ensuring product safety.
What is Quorum Sensing?
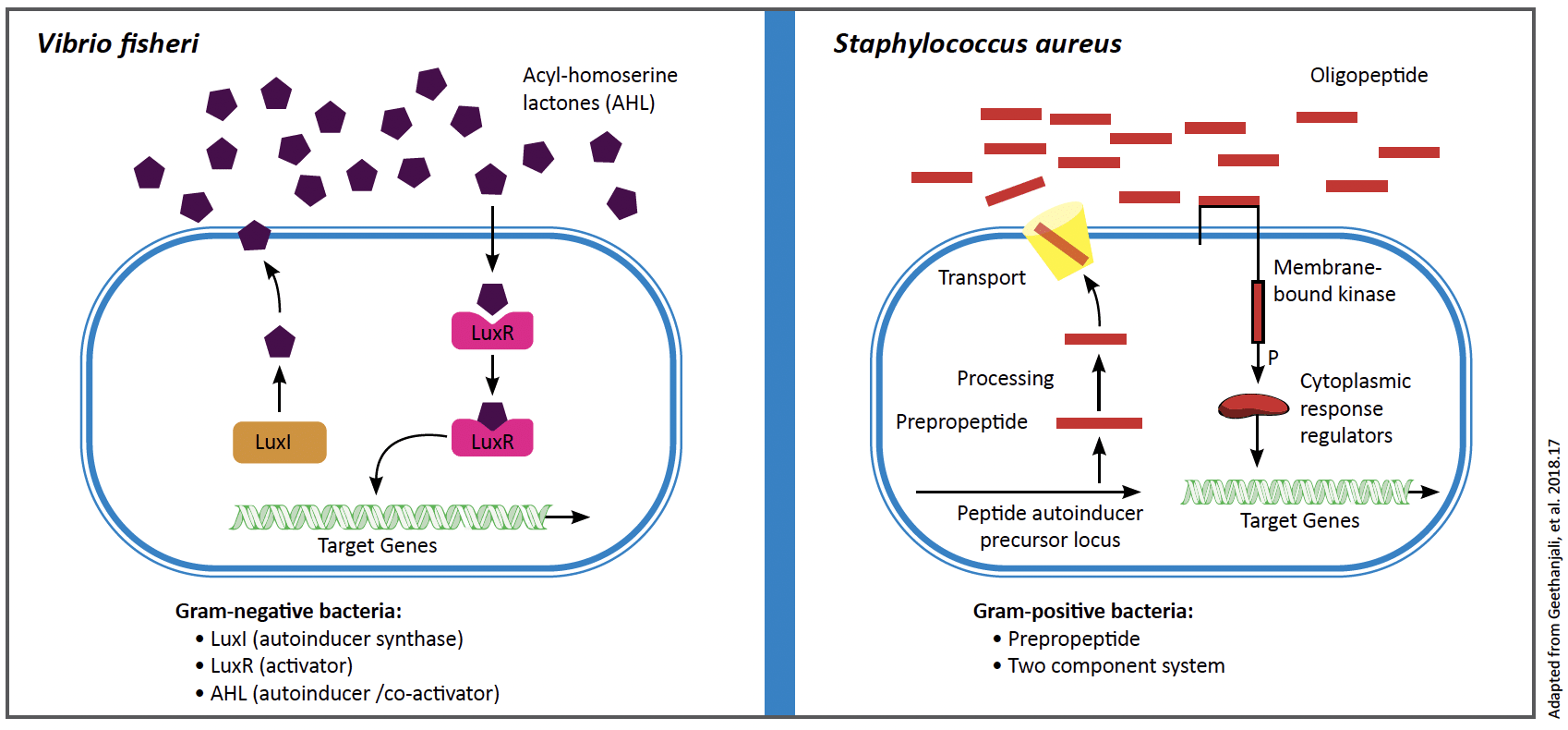
Quorum sensing is a cell-to-cell communication system that enables bacteria to regulate gene expression in response to fluctuations in population density and environmental stress. The significance of this process cannot be overstated. The primary players in this system are signaling molecules called autoinducers, which are synthesized and secreted by bacterial cells. As the bacterial population grows, the concentration of autoinducers increases until it reaches a critical threshold at which the concentration outside the cell is greater than inside the cell. At this point, the autoinducers follow the concentration gradient to move into the cell. This allows them to bind to specific cell receptors and trigger coordinated gene expression changes.
The mechanisms of QS vary among bacterial species. Gram-negative bacteria typically use acyl-homoserine lactones (AHLs) as their autoinducers. These are detected by the LuxR receptor once a threshold concentration is reached.1 On the other hand, Gram-positive bacteria often rely on oligopeptides as signaling molecules. These activate two-component signal transduction systems consisting of a sensor kinase and a response regulator.2 Autoinducer-2 (AI-2) is another signaling molecule that enables interspecies communication among both Gram-negative and Gram-positive bacteria.1
Quorum Sensing and Biofilm Formation
Biofilm formation is a critical collective behavior regulated by QS. Forming biofilms is crucial for bacteria to establish stable colonies on surfaces in food processing equipment, where the biofilm shields them from environmental stressors such as disinfectants, antibiotics, and extreme temperatures. Once a biofilm is established, QS regulates the production of extracellular polymeric substances (EPS), which form the structural matrix that protects the embedded bacterial community.
For instance, in Pseudomonas aeruginosa, QS regulates the production of alginate, a polysaccharide that is a key component of the biofilm matrix.3 Quorum sensing systems in this bacterium, such as the las and rhl systems, also control the production of rhamnolipids, biosurfactants that contribute to the biofilm’s architecture.4 Similarly, Vibrio cholerae uses QS to control the production of its biofilm matrix component, Vibrio polysaccharide (VPS), allowing the biofilm to disperse once the population density reaches a certain level.5
In food processing environments, biofilms can form on a variety of surfaces, such as stainless steel, Teflon, plastic, and rubber, and are notoriously difficult to remove once established.6 The EPS matrix provides structural integrity to the biofilm and creates a microenvironment that protects the bacteria from environmental stressors such as disinfectants, reactive oxygen species (ROS), and pH fluctuations.4 This ability of biofilms to persist and resist cleaning protocols poses a major threat to food safety and quality.
Quorum Sensing and Resistance to Environmental Stressors
Quorum sensing plays a central role in enhancing the resistance of biofilms to environmental stressors, including antimicrobial agents commonly used in food processing. Bacteria within biofilms are more resistant to disinfectants due to the protective EPS matrix and the upregulation of resistance genes triggered by QS. For instance, QS can induce the expression of efflux pumps, which actively expel certain antimicrobials from bacterial cells, reducing their intracellular concentrations and conferring resistance. Similarly, they can induce the expression of proteins that inactivate antimicrobials before they have a chance to engage the cell.5
In Listeria monocytogenes, a common foodborne pathogen, for example, QS has been shown to regulate the expression of stress response genes that confer resistance to oxidative stress, including catalase and superoxide dismutase, enzymes that detoxify ROS produced by disinfectants.3 Additionally, QS can regulate the production of enzymes such as β-lactamases, which degrade antibiotics before they can reach their targets.4
Implications for Food Quality and Safety
The persistence of biofilms in food processing environments has significant implications for food quality and safety. Biofilms serve as reservoirs for pathogenic and spoilage bacteria that can intermittently detach and contaminate food products. This is particularly concerning in ready-to-eat (RTE) products, including dairy and beverage products, where post-processing contamination can lead to foodborne outbreaks and spoilage.6 Biofilms can also facilitate cross-contamination within a processing facility, as bacteria can disperse from biofilms, spread to other areas, and form new biofilms.6
Furthermore, QS-regulated enzymes and secondary metabolites can contribute to food spoilage by degrading food components and producing undesirable flavors and odors through the expression of proteases, lipase, and sulfur compounds.7 The presence of biofilms on processing equipment can shorten the shelf life of food products by introducing spoilage organisms that continue to metabolize food components during refrigerated storage after packaging.8 This leads to economic losses and diminishes consumer confidence in the brand.
Mechanisms of Bacterial Spoilage
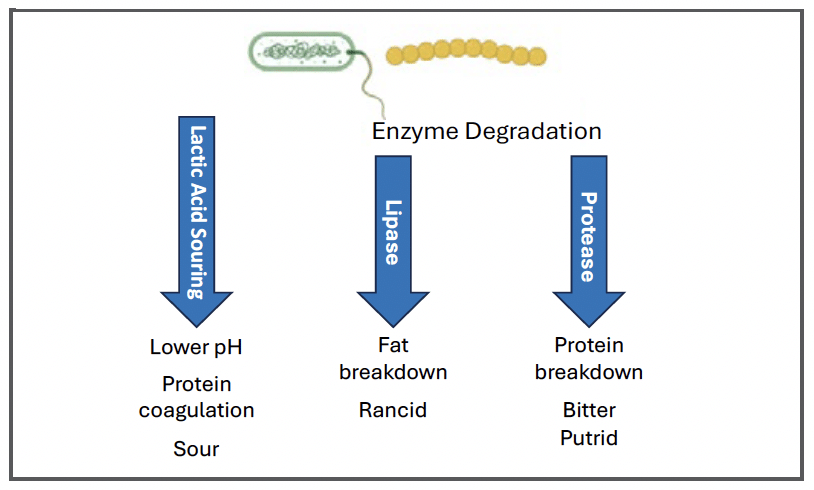
1) Lactic acid souring; 2) breakdown of fats by lipase enzymes; and 3) breakdown of proteins by protease enzymes. Each causes characteristic flavor, odor, and textural change to the milk.
The Potential for Controlling Biofilms through Quorum Sensing Inhibition
Given the significant role that QS plays in biofilm formation and resistance, disrupting QS through quorum sensing inhibitors (QSIs) has emerged as a promising strategy for controlling biofilms in food processing environments. Quorum sensing inhibitors can block the synthesis or reception of autoinducers, preventing the coordinated expression of biofilm-related genes. For example, halogenated furanones, naturally occurring compounds found in some red algae, have been shown to interfere with AHL-based QS systems, reducing biofilm formation in Pseudomonas aeruginosa and other Gram-negative bacteria.3
Several plant-derived compounds, such as cinnamaldehyde (from cinnamon) and vanillin (from vanilla), have also been identified as potential QSIs. Research has demonstrated that these compounds can inhibit biofilm formation by Escherichia coli and Salmonella enterica in food-related environments by interfering with the AI-2 signaling pathway.9Synthetic QSIs are also being developed to block QS more effectively, offering a more robust approach to controlling biofilms.10
The Importance of Process Monitoring as Current Best Practice
Despite the potential of QSIs to disrupt bacterial communication and prevent biofilm formation, these technologies are not yet widely available for use in the food and dairy processing industries. Consequently, stringent process monitoring remains essential for detecting and managing biofilms in these environments. Monitoring systems such as the QualiTru TruStream aseptic sampling system provide critical insights into the microbiological status of liquid food and dairy processes. Regular sampling allows for the early detection of biofilm formation and microbial contamination, enabling processors to take timely corrective actions before contamination reaches harmful levels.4
By integrating vigilant process monitoring with effective cleaning and sanitization practices, food processors can mitigate the risks associated with biofilms, even in the absence of QSIs. The ongoing development of monitoring technologies will continue to play a pivotal role in enhancing food safety and quality, ensuring that biofilms remain a manageable challenge rather than a persistent threat.4
Suggestions for Controlling and Preventing Biofilms
- Implement Regular Process Monitoring: Use aseptic sampling systems to frequently monitor key points in the process for early detection of biofilm formation.
- Optimize Cleaning Protocols: Ensure that cleaning and sanitization procedures target biofilm-prone areas, including hard-to-reach surfaces and equipment crevices.
- Use Biofilm-Specific Sanitizers: Incorporate cleaning agents specifically designed to penetrate and break down biofilm matrices, such as enzymatic cleaners or disinfectants that target EPS.
- Control Surface Roughness: Minimize surface irregularities on equipment (like welds, gaskets, or valves) where biofilms are more likely to form by ensuring smooth and cleanable surfaces.
- Disrupt Quorum Sensing: Explore the use of QSIs to block bacterial communication, preventing the formation and maintenance of biofilms.
- Increase Frequency of Sanitation: Conduct more frequent cleaning cycles, especially in areas prone to biofilm formation, to prevent biofilms from becoming established.
- Rotate Cleaning Agents: Use different types of cleaning agents in rotation to avoid bacteria developing resistance to specific sanitizers.
- Maintain Optimal Process Conditions: Monitor and control factors such as temperature, pH, and moisture levels to create an environment less conducive to biofilm development.
- Use Mechanical Cleaning: Incorporate mechanical action, such as brushing or scrubbing, with cleaning agents to physically remove biofilms from surfaces.
- Train Employees on Biofilm Risks: Provide thorough training for staff on the risks of biofilms and best practices for cleaning and maintenance to prevent their formation.
Harnessing the Power of Quorum Sensing: The Future of Biofilm Formation Control in Food Safety
Quorum sensing is a fundamental mechanism that regulates biofilm formation, EPS production, and resistance to environmental stressors in bacterial communities. In the context of food processing, QS contributes to the persistence and resilience of biofilms on equipment surfaces, posing significant challenges to food quality and safety. While QSIs represent a promising direction for mitigating biofilm risks, stringent process monitoring and effective cleaning protocols remain essential for controlling microbial contamination in food and dairy processing environments.
The development of new strategies that integrate QSIs, EPS-degrading enzymes, and enhanced sanitization protocols will be key to overcoming the challenges posed by biofilms and safeguarding food safety in the future.
Take control of biofilm risks in your processing environment. Download our QS white paper for a deeper dive into how bacteria communication shapes biofilms or contact us at (651) 501-2337 to learn more about integrating QualiTru aseptic sampling systems into your operations to help ensure food safety and quality.
References
1. Waters CM, BL Bassler. Quorum sensing: cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology, 2005; 21, 319-346.
2. Fuqua C, EP Greenberg. Listening in on bacteria: acyl-homoserine lactone signaling. Nature Reviews Molecular Cell Biology, 2002; 3(9), 685-695.
3. Hentzer M, H Wu, JB Andersen, K Riedel, TB Rasmussen, N Bagge, ... M Givskov. Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. The EMBO Journal, 2003; 22(15), 3803-3815.
4. Flemming HC, J Wingender. The biofilm matrix. Nature Reviews Microbiology, 2010; 8(9), 623-633.
5. Rutherford ST, BL Bassler. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor Perspectives in Medicine, 2012; 2(11), a012427.
6. Skandamis PN, GJE Nychas. Quorum sensing in the context of food microbiology. Applied and Environmental Microbiology, 2012; 78(18), 6373-6381.
7. Natrah FMI, T Defoirdt, P Sorgeloos, P Bossier. Disruption of bacterial quorum sensing: An unexplored strategy to control aquatic bacterial diseases. Aquaculture, 2011; 317(1-4), 1-9.
8. Rasch M, C Buch, B Austin, L Gram. Multiple quorum sensing inhibitors reduce virulence in a fish pathogen. Applied and Environmental Microbiology, 2004; 70(8), 4651-4656.
9. Nazzaro F, F Fratianni, L De Martino, R Coppola, V De Feo. Effect of essential oils on pathogenic bacteria. Pharmaceuticals, 2013; 6(12), 1451-1474.
10. Adonizio A, KF Kong, K Mathee. Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa by South Florida plant extracts. Antimicrobial Agents and Chemotherapy, 2008; 52(1), 198-203.
11. Geethanjali DK, V Kumar, N Raghu, TS Gopenath, S Veerana Gowda, … M Kanthesh. Quorum sensing: A molecular cell communication in bacterial cells. Journal of Biomedical Sciences, 2018; 5(2):23-34.