Ingredient Quality Assurance Starts with Confidence
Ingredient quality assurance begins before production and forms the foundation of every finished food and beverage product. From dairy bases to syrups, edible oils, nutraceuticals, and flavorings, producers rely on a steady flow of raw materials that meet strict specifications. Yet sampling ingredients directly from their transport containers, such as drums, totes, carboys, and intermediate bulk containers (IBCs), poses challenges. Opening containers to collect samples exposes ingredients to oxygen, moisture, and airborne contaminants. These risks can compromise sensitive products and leave processors with incomplete or unreliable data for assessing safety, consistency, and compliance.
Aseptic, closed-system sampling eliminates these risks. By enabling non-invasive access to containers, processors can verify ingredient quality without disrupting the product environment.
Our organization is pleased to have partnered with QualiTru Sampling Systems in testing their closed-system, drum sampling cap for maple syrup barrels. We are confident that these innovations will offer substantial benefits to both maple syrup producers and packers by reducing the time needed to collect samples and the spoilage risks associated with contaminants.
Luc Goulet, President of the Quebec Maple Syrup Producers.
The Hidden Costs of Poor Visibility
Food and beverage manufacturers recognize the importance of ingredient quality, but poor sampling practices can undermine the very assurance they are intended to provide. Dipper, open-container, and valve sampling methods increase risk of pulling samples that are contaminated or unrepresentative of the container as a whole. Additionally, improper/poor sampling practices with dipper and open-container methods also risk contamination of the entire product. Such practices may also expose sensitive ingredients to oxygen, moisture, or environmental contaminants, unintentionally creating new risks.
Aseptic, closed-system sampling helps improve the accuracy of data used for quality decisions and certificate of analysis (COA) verification by reducing the likelihood of incomplete or misleading results.
Inaccurate results lead to a false sense of security that ultimately can result in:
- Wasted product: Entire batches may need to be discarded or recalled when quality issues surface late in production or after shipping.
- Operational inefficiencies: Labor-intensive sampling practices consume time and increase handling risks.
- Compliance and audit risks: Data collected without validated methods and documented COA verification may not withstand regulatory or audit scrutiny.
- Allergen exposure: Inadequate or inconsistent methods complicate allergen verification and segregation efforts.
- COA gaps: Supplier COAs document the expected specifications of a lot, but only closed-system aseptic verification sampling can confirm the container’s actual specifications.
Frequently Asked Questions Ingredient Sampling for Food and Beverage Quality Control
Q: What is ingredient quality assurance?
Ingredient quality assurance is a structured program that verifies the safety, integrity, and consistency of incoming materials through sampling, testing, documentation, and supplier management.
Q: How does closed-system sampling help?
Closed-system sampling prevents exposure during sample collection, provides representative data, and supports HACCP/FSMA/GFSI verification including supplier verification for Supplier Preventive Controls and GFSI Quality Controls.
Q: Which containers can be sampled with a closed-system method?
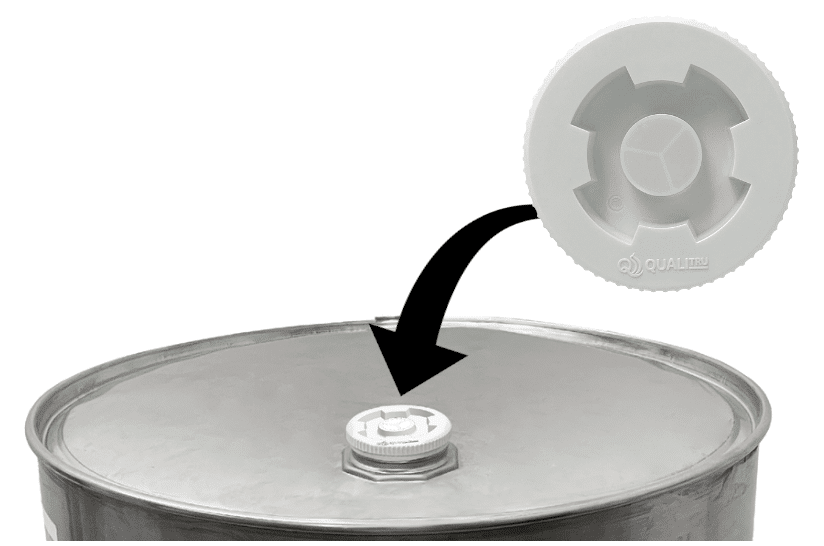
Closed-system sampling can be applied to drums, totes, intermediate bulk containers (IBCs), and other sealed ingredient containers using devices such as the TruStream3™ NPS Drum Sampling Cap with integrated septum.
Q: What does “aseptic sampling” mean?
Aseptic sampling is the collection of a sample using a sterile device and validated procedures that prevent the introduction of outside microorganisms. The sample may contain microbes from the ingredient, but aseptic technique ensures those microbes reflect only the product, not contamination introduced during sampling.
Q: Why not just rely on a supplier’s certificate of analysis (COA)?
A COA confirms what the supplier intends to provide, but it does not verify the actual contents of each container. Closed-system aseptic sampling provides direct evidence of ingredient quality, ensuring that COA documentation matches reality.
Q: How does closed-system sampling support food and beverage quality control?
By verifying raw material quality before use, closed-system aseptic sampling reduces variability, contamination risk, and labeling errors—improving both product consistency and regulatory compliance.
Q: What are the cost implications of better sampling?
Reliable sampling prevents costly recalls, rework, and product waste by identifying quality issues before production begins. It also reduces audit risk, saving time and resources associated with compliance challenges.
Have questions about closed-system sampling for ingredient quality assurance?
Need a quote? We’ll respond to
your request quickly.
Looking for one of our global distribution partners near you?
Closed-System Ingredient Sampling: A Smarter Approach
Closed-system aseptic sampling allows processors to monitor ingredient quality without breaking container seals. By protecting product integrity, this method helps ensure that the data collected is both accurate and actionable.
The advantages extend across the plant:
- QA/QC teams acquire samples that are free from cross-contamination or allergen cross-contact and reliably reflect true product quality.
- Operations managers reduce waste, downtime, and rework.
- Plant leadership gains confidence in compliance, efficiency, and customer satisfaction.
- Suppliers and logistics teams maintain product value across the supply chain.
Closed-system sampling turns ingredient containers from potential blind spots into active quality assurance checkpoints.
Applications Across the Food and Beverage Spectrum
The advantages of container sampling are not limited to one product or sector. Across the food and beverage industry, aseptic, representative sampling safeguards product quality without introducing new risks through oxidation, contamination, or disturbance of product layers.
- Dairy bases and dairy processing: Aseptic microbiological sample collection helps processors confirm ingredient quality before blending or bottling, ensuring early visibility into hygiene and safety.
- Infant nutrition: Closed-system sampling supports regulatory compliance through verifiable aseptic methods ensuring that reliable samples are collected from high-value liquid bases where accuracy and safety are non-negotiable.
- Syrups and sweeteners: Closed-system, aseptic sampling methods allow processors to reliably test Brix, microbial load, and color without opening containers.
- Edible oils oxidation and moisture ingredients: Sampling without oxygen exposure preserves product integrity.
- Nutraceuticals and functional foods: Controlled, aseptic sampling safeguards bioactive ingredients and provides traceable data for compliance with HACCP and ISO standards.
- Fermented beverages: Sampling under closed conditions helps monitor microbial activity and pH while protecting fermentation stability.
- Pharmaceutical-grade ingredients: Verifiably aseptic sampling methodology supports traceability, regulatory documentation, and assurance of ingredient quality during storage and transport.
By replacing traditional, open methods with a closed-system approach that ensures aseptic and representative sample collection, processors gain trustworthy data, safeguarding both product quality and brand reputation.
Take Control of Ingredient Quality
Ingredient sampling should provide answers, not add risk. With hygienic, non-invasive, closed-system access to drums, totes, and other containers, processors can reduce waste, meet regulatory requirements, and safeguard product quality from the start.
Email [email protected] to learn more or call us at (651) 501-2337 to talk to a QualiTru expert today to learn how container sampling can strengthen your quality and safety programs.



