Maintaining the Quality of Pasteurized Fluid Milk
Quality control of pasteurized fluid milk can be complex and requires the understanding of microbial contamination, both in raw and processed milk. Aseptic and representative sampling plays an important role in monitoring raw milk quality and process monitoring for contamination within the dairy plant.
Fluid milk (A commonly used term in the industry for milk processed for beverage use) continues to remain an important part of the balanced diet according to the MyPlate Plan published by the Dietary Guidelines for Americans. All dairy goodness begins with milk production, where the focus is on bringing nutritious, delicious, and safe products to the marketplace.
Improving the quality of fluid milk by pasteurization
 Pasteurization of raw fluid milk was introduced in the United States in the 1890s to control the hazards of highly contagious bacterial diseases that could be easily transmitted to humans through the drinking of raw milk. (Source: Wikipedia®).
Pasteurization of raw fluid milk was introduced in the United States in the 1890s to control the hazards of highly contagious bacterial diseases that could be easily transmitted to humans through the drinking of raw milk. (Source: Wikipedia®).
Pasteurizing has made great strides over the past 100+ years when a New Jersey milk plant installed the first pasteurizer in the United States. Today, all milk in the United States is pasteurized with the exception of a small percentage that is sold as “raw” milk.
The pasteurization process is one of the many ways the U.S. dairy industry helps ensure that our milk supply is safe for consumption.
Several factors can impact the quality of pasteurized fluid milk, including milk fat oxidation, elevated somatic cell counts, and off-flavors from cows’ feed. The most significant impact on quality is microbial spoilage.
There are two types of microbial spoilage that can occur:
- Heat-resistant psychotropic bacteria that usually originate from raw milk (This is a topic for a separate blog post in the future).
- More frequent and significant to quality is post-pasteurization contamination within the plant, which is most often linked to gram-negative bacteria.
Bacteriological standards in raw vs pasteurized milk
The chart below shows the standards for Grade “A” milk (Source: “Basic Dairy Bacteriology” published by the Department of Food Science at Cornell University)
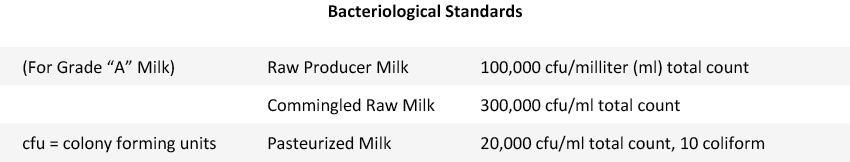
Pasteurization (161°F for 15 sec.) is designed to destroy most potential pathogens in raw milk which substantially reduces bacterial counts for most freshly pasteurized milk to less than 500 cfu/ml which is far lower than the standard of 20,000 cfu/ml.
Unless proper preventive measures are taken at the dairy processing plants, contamination can occur as post-pasteurization contaminants (PPC). This post outlines below some of the sources and measures that must be taken to prevent microbial contamination.
How dairy plants identify and minimize the risk of contamination
Dairy plants that process fluid milk must routinely test and evaluate their products for two important reasons:
- Regulatory Compliance—This includes lab pasteurized counts, Coliform counts, and initial standard plate counts.
- Quality and Safety—Consumer acceptance and trust in the quality and safety of the dairy products they consume.
A plant sanitation program and verification of process control are critical to minimizing microbial spoilage. Risks can include cracked pasteurizer plates or product storage tanks, and airborne contaminants, as well as ineffective personal hygiene.
Compressed air is often a source of contamination if compressed air dryers are functioning improperly. All risks need to be monitored and controlled.
Importance of aseptic and representative sampling in process monitoring
QualiTru has a proven method for aseptic and representative sampling used for process monitoring in plants worldwide. The National Conference on Interstate Milk Shipments (NCIMS) and the Food & Drug Administration (FDA) have approved QualiTru sampling products for collection of the milk producer’s “Universal” Dairy Farm Milk Samples under Section 6 of the Grade “A” Pasteurized Milk Ordinance (PMO). (See Regulatory Approvals).
The QualiTru sampling system emphasizes the importance of sampling at critical control points throughout the process. When coupled with standard approved laboratory methodologies, this sampling method can be used to identify the sources of contamination. Aseptic sampling and aseptic testing techniques are both necessary for processors to have confidence in their data.
As we like to say around here, “Your Test Result is Only as Accurate as Your Sample.”
How does microbial testing determine contamination sources in pasteurized fluid milk?
The Mosely Keeping Quality Test is one of several industry-standard tests for the detection of microbial spoilage and other defects. The test requires pasteurized milk to be held for seven to ten days at 45 degrees F., tasted for flavor, and then plated for bacteria. Bacteria counts in the millions per milliliter indicate the need for further analysis.
Gram-staining from the plate counts can identify bacterium types and help further verify the source of spoilage. Post-pasteurization contamination can include both gram-negative and gram-positive bacteria. Gram-positive bacteria could be a potential, though unlikely source, of post-pasteurization contamination.
The presence of any gram-negative bacteria typically indicates contamination in the process. Most psychotropic bacteria are gram-negative and grow at a faster rate at refrigeration temperatures; their presence is most likely due to post-pasteurization contamination. In addition to the spoilage potential of gram-negative bacteria, their presence can also indicate the potential for pathogenic bacteria, which can result in food safety concerns.
Read our case study on Sanitation Verification Using Aseptic Sampling.
Have questions about aseptic and representative sampling? Ask Our Experts