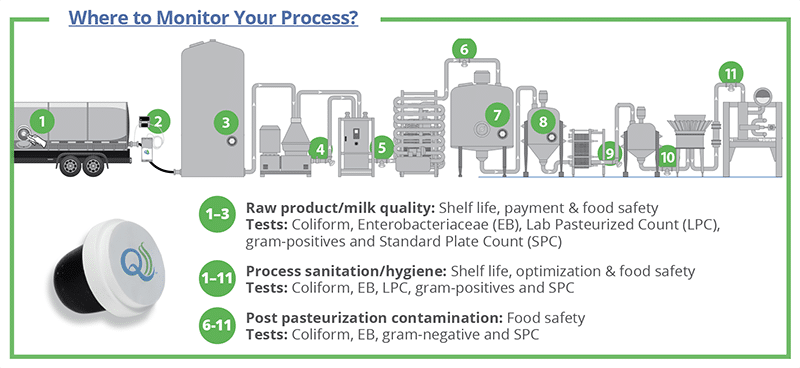
Why is Aseptic Inline Sampling Critical to Process Monitoring?

Recent dairy recalls, ranging from Listeria outbreaks in ice cream to Cronobacter in infant formula, and the Food and Drug Administration’s (FDA’s) updated Investigations Operations Manual underscore the importance of inline process monitoring for bacterial risks. Alongside environmental monitoring, aseptic inline sampling at pasteurizers, silo tanks, and other key points helps teams detect issues sooner, verify sanitation, and protect product quality and shelf life.
The FDA defines aseptic sampling as using sterile implements and containers so the product only contacts the sampling tool or container. Samples collected this way accurately reflect the condition of the lot at the time of sampling and, ideally, at shipment. The FDA also notes that aseptic technique is critical for microbiological testing and for chemical analyses that could be altered by microbial activity. Where applicable, shipping conditions should keep microbes inactive to prevent degradation of the analyte. Aseptic sampling is routinely applied to inline, environmental, bulk product, and unpackaged product samples.
Quality managers, engineers, and producers often face unclear responsibilities and procedures across the process chain. A proactive approach, such as choosing the right sampling method and standardizing when and where to sample, supports faster root-cause analysis, fewer holds and reworks, and stronger process control.
Aseptic Inline Sampling Is Critical for Accurate Test Results
The sampling method you choose drives data integrity. Aseptic inline sampling preserves microbial integrity and yields more representative results than open grabs or ad-hoc valves. QualiTru Sampling Systems® provides closed-system, aseptic access so teams can collect samples during production without introducing contamination or stopping the line.
Aseptic process monitoring involves installing a series of QualiTru stainless steel ports at critical points along the line. The QualiTru TruStream Septum, with either seven or 12 individual channels, is designed for single samples to be drawn from each channel — dramatically reducing the risk of cross-contamination. Each septum is made of high-quality, food-grade materials and features a label that clearly indicates which channels have been used. Once all channels have been used, simply dispose of the septum and replace it with a new one.
Closed-system vs Grab/Dipper Sampling
Here is a quick comparison of common sampling methods to help standardize your plan and protect data integrity.
| Method | Microbial integrity | Representativeness | Labor/complexity | Common risks |
|---|---|---|---|---|
| Inline aseptic (closed system) | High | Reflects actual flow conditions | Moderate | Requires planned port placement |
| Grab or dipper | Low to variable | Moment-in-time, operator-dependent | Low | Exposure, bias, false highs or lows |
| Time-based composite | High if container is sterile | Time-averaged view for payment and trends | Moderate | Container sterility and handling errors |
Takeaway: Use inline aseptic for process verification. Use composite for payment and long-run trends. Avoid open grabs when data integrity matters.
How the QualiTru Sampling System Works
QualiTru collection units use a sterile needle and tubing to create a closed, aseptic fluid path for sample collection. For continuous flow sampling, route the tubing through an electric or portable peristaltic pump so a representative composite can be collected over the entire run. Coupled with documented aseptic technique, this closed system lowers the risk of inaccurate or unreliable results. Aseptic technique requires procedures that reduce exposure to microorganisms and keep tools and contact surfaces as free from contamination as possible. These steps are defined in our standard operating procedures (SOPs).
Challenges of Non-aseptic Sampling
- Micro ports: Micro ports are non-sterile grommets that may introduce contamination into the sample due to a lack of distinct channels. This may introduce gram-positive bacteria because the port is used more than once, and the product sits in the grommet over time.
- Valves: Valves require steam to kill all spore-forming bacteria. They also require additional energy and labor that may add up to 20 minutes per valve for proper clean-out-of-place (COP) or steam sterilization. Cleaning a non-sterile port requires disposing of the product that is in the valve to ensure it does not contaminate the sample. One customer estimated 50,000 Euros in cumulative loss due to the wasting of product from 15 seconds of flushing the valves across ten silos over a year.
What Are Other Key Components for Effective Inline Process Monitoring?
Target gram-negative indicators for post-pasteurization contamination
Gram-negative bacteria do not generally survive pasteurization, so detecting their presence in process monitoring samples is a warning sign of post-pasteurization contamination that can lead to spoilage and food safety issues. Their presence in pasteurized products indicates contamination somewhere along the process. Even very low levels, often < 1 bacterium per gallon, can grow at refrigeration temperatures and cause premature spoilage. More importantly, gram-negative bacteria could also indicate foodborne pathogens, such as Salmonella, E. coli, or Cronobacter. A positive post-pasteurization result requires corrective action.
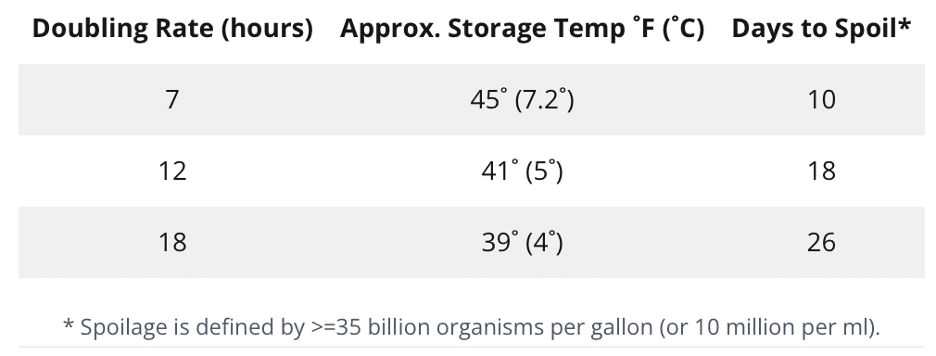
Psychrotrophic growth and generation time
Even small numbers of gram-negative psychrotrophs can matter because they multiply at cold temperatures. Generation time depends on organism, oxygen, temperature, and nutrients. Milk is nutrient-rich, so growth accelerates as temperature rises, with the fastest growth near room temperature. Use inline sampling and trending to catch early changes, and verify cooler logs and time-temperature exposures.
Look beyond total bacterial counts
Total counts alone can miss early post-pasteurization contamination signals. Focus on which organisms you find and where you find them, then trend aerobic plate counts (APC), coliforms, and spores by sampling location to localize issues and confirm control after corrective actions. Effective process monitoring must focus on the type rather than the number of bacteria.
Psychrotrophic growth and generation time in pasteurized milk
If You Cannot Measure Contamination Accurately, You Cannot Control It!
Accurate, representative sampling paired with environmental monitoring gives teams reliable trends for faster corrections and better risk control. As we like to say around here… Your Test Result Is Only as Accurate as Your Sample.
How can we help?
Do you have a contamination issue you are having trouble solving or would you like to discuss a proactive process monitoring plan? Complete this form to have one of our experts contact you for a FREE consultation. Or call us at (651) 501-2337 or email [email protected] to learn more and/or to discuss your needs.



