Whey Processing: How Biofilms Silently Sabotage Extended Runs
Whey processing during cheesemaking is a balance of biological precision, equipment design, and time-sensitive process control. While modern production facilities have mastered the major threats to quality and efficiency, even in the most advanced plants, one subtle but insidious threat continues to compromise extended production runs: biofilms in the whey processing line. These resilient microbial communities, forming deep within pipelines and fittings, pose an underappreciated risk to yield, product quality, and overall hygiene. When overlooked, they quietly sabotage operations by shedding intermittent contamination that is undetectable by routine monitoring but potent enough to erode quality and consistency.
What Are Biofilms and Why the Whey Processing Line?
Biofilms are structured communities of microorganisms encased in a self-produced matrix of extracellular polymeric substances (EPS), which allows them to adhere tightly to surfaces. Once formed, biofilms become highly resistant to cleaning and sanitizing agents, especially in areas of low turbulence or stagnant flow (Branda et al., 2005). While biofilm risks are widely acknowledged in areas like pasteurizers and filler heads, the whey line receives far less attention despite its vulnerability.
Whey, which is rich in residual proteins, lactose, and moisture, is a prime nutrient source for bacterial proliferation. During extended whey processing runs, especially with high-yield cheeses like Mozzarella or Cheddar, whey collection lines transport significant volumes of warm, nutrient-laden fluid over many hours. This prolonged exposure and temperature window (often 25–40°C or 77–104°F) can accelerate microbial adhesion and biofilm development. According to Sharma and Anand (2002), proteinaceous materials in dairy streams facilitate rapid bacterial attachment, particularly in stainless steel surfaces exposed to intermittent flow.
How Biofilms Form During Extended Runs
Extended runs are designed to optimize throughput and minimize downtime, but they also lengthen the exposure window during which bacteria can settle and grow in suboptimally cleaned regions. In the whey processing line, vulnerable zones include:
- Pipe joints with gaskets or micro-gaps
- Low-velocity sections, elbows, or horizontal piping runs
- Collection manifolds and flow splitters
- Sight glasses or sensor housings
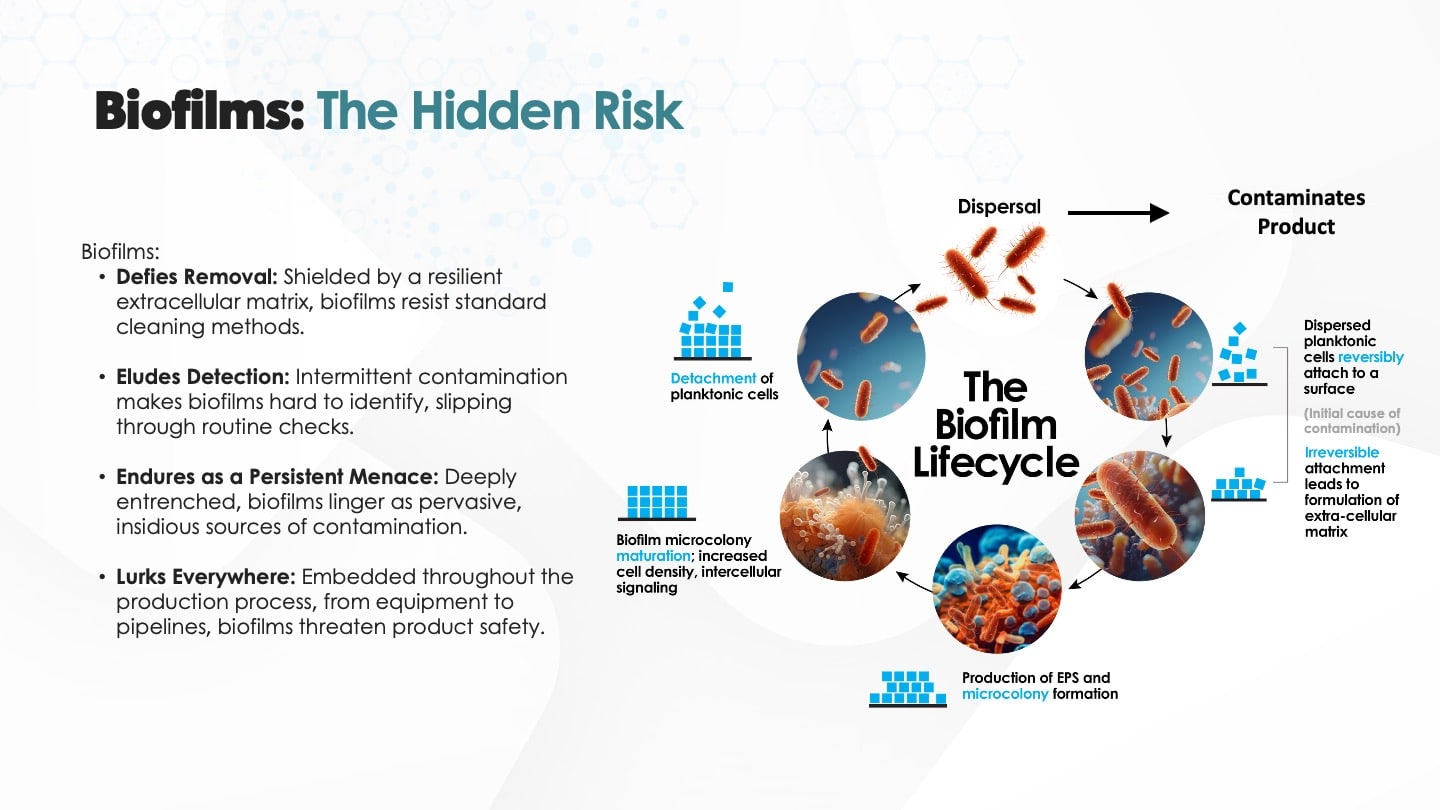
Bacteria that survive standard clean-in-place (CIP) cycles, such as thermoduric or spore-forming organisms, can anchor themselves in these crevices. Over time, as whey passes over the colonized zone, nutrients feed the colony and the biofilm expands. This is not an even or predictable process. Biofilms mature in stages, and once mature, they periodically release cells or clumps of biomass in a process known as sloughing, which then contaminate downstream product or contact surfaces (Flint et al., 1997).
This contamination is rarely continuous. Instead, it’s stochastic, intermittent, and difficult to predict. That makes it harder to catch using traditional testing at fixed intervals or endpoints. As a result, by the time microbial spikes appear in whey quality metrics or finished product sampling, the contamination has already occurred.
Impacts of Biofilms on Whey Processing Quality and Yield
Biofilms in the whey processing line can impact production in several ways:
- Spore and Thermoduric Contamination: These organisms survive pasteurization and contribute to product spoilage, off-flavors, or shortened shelf life in whey powders and concentrates.
- Rework and Hold Decisions: Inconsistent microbial counts in whey streams can lead to downstream hesitation, triggering product holds, reruns, or filtration steps that reduce efficiency.
- Reduced Shelf Life or Stability: In cheeses where whey is partially retained (e.g., high-moisture Mozzarella), even trace contamination can lead to surface defects, off-odors, or reduced storage stability.
- Risk of Cross-Contamination: Biofilms in the whey line can seed organisms into CIP return flows or shared lines, contaminating areas thought to be isolated.
Even when not producing defects detectable at the sensory level, these microbial incursions reduce confidence in sanitation efficacy and complicate root cause investigations during quality drift.
Traditional Monitoring Falls Short
Routine microbial monitoring in cheesemaking often focuses on raw milk, final product, and environmental testing, such as surface swabbing. But these approaches can easily miss transient contamination events. Swabbing after cleaning may return acceptable Adenosine Triphosphate (ATP) results yet fail to detect live biofilm communities protected under EPS layers (Sela et al., 2022).
Similarly, endpoint sampling of whey, for example at the silo or outlet, provides only a snapshot, often taken after dilution or blending has masked the initial contamination. The dynamic nature of biofilm sloughing means that even hourly composite samples may miss peak events.
This is particularly concerning in operations with long whey runs. In such environments, contamination introduced in hour three may not appear until hour five, and then only in trace amounts. Without better in-line visibility, biofilms continue to grow unchecked.
In-Process Sampling as a Proactive Defense
To detect biofilm presence before it manifests as a defect, in-process sampling is essential. By installing sterile sampling ports at key locations, such as immediately after curd separation, midway through the whey line, and near the discharge point, processors can gain a temporal profile of microbial activity.
Sampling whey at these points allows Quality Assurance (QA) teams to:
- Identify microbial spikes before they reach end-of-line concentrations
- Track fluctuation patterns across multi-hour runs
- Compare microbial loads across line segments to pinpoint biofilm hot spots
This type of temporal and spatial mapping offers a way to move from reactive to predictive QA. If spikes consistently appear mid-run, in-process data can drive maintenance decisions such as mid-shift cleaning, gasket replacement, or line redesign.
In-process sampling also supports CIP validation. By testing the same points pre- and post-CIP, processors can assess the effectiveness of their cleaning cycles over time and adjust concentrations, flow rates, or cleaning durations as needed.
Designing for Prevention
Eliminating biofilm risks in the whey line begins with equipment design. Piping and fittings should follow 3-A Sanitary Standards and be installed with hygienic principles in mind:
- Maintain adequate flow velocities (typically >5 ft/sec)
- Avoid horizontal runs without slope
- Eliminate dead legs and ensure full drainability
- Use self-draining valve and instrumentation designs
Beyond design, operational policies should support shorter runs between cleaning, scheduled mid-run sanitation for high-risk products, and enhanced cleaning validation using biofilm-specific methods.
Upgrades to sampling infrastructure also make a difference. Traditional valve sampling or manual dip methods increase contamination risk and reduce precision. Aseptic septum-based sampling systems, such as those provided by QualiTru, enable closed, representative collection from pressurized or CIP-active lines without compromising sterility.
Proactively Manage Biofilm Risks in Your Whey Processing Line
Biofilms in the whey processing line are a hidden threat, especially during extended production runs. Their ability to develop unnoticed and shed contaminants intermittently makes biofilm control uniquely difficult using standard monitoring tools. For cheesemakers focused on yield optimization and quality assurance, this silent saboteur can quietly undermine performance over time.
In-process sampling offers a powerful tool to detect biofilm activity early, validate cleaning protocols, and guide process improvements. Coupled with hygienic equipment design and microbial awareness, it gives QA managers the insight they need to protect product integrity and operational efficiency.
Ready to tackle biofilms and protect your whey processing lines?
We offer two convenient ways to request a quote:
- Request for Quote (RFQ): Visit our product pages to select items and submit an RFQ. A QualiTru representative will follow up promptly with pricing and ordering information.
- Custom Quote Form: Use our Custom Quote Form to provide details about your specific operation and the challenges you’re facing. Our experts will then reach out directly to recommend the optimal sampling system configuration for your process.
You can also call us at (651) 501-2337 or email [email protected] to learn more and/or to discuss your needs.
References:
Branda, S. S., Vik, Å., Friedman, L., & Kolter, R. (2005). Biofilms: the matrix revisited. Trends in Microbiology, 13(1), 20–26. https://doi.org/10.1016/j.tim.2004.11.006
Flint, S. H., Brooks, J. D., & Bremer, P. J. (1997). The influence of cell surface properties of thermophilic streptococci on attachment to stainless steel. Journal of Applied Microbiology, 83(4), 508–517.
Sela, S., Amir, I., & Pinto, R. (2022). Biofilm formation and cleaning efficiency in dairy processing lines: Risks and control strategies. Journal of Dairy Science, 105(4), 3278–3290.
Sharma, M., & Anand, S. K. (2002). Biofilms evaluation as an essential component of HACCP for food/dairy processing industry–a case. Food Control, 13(6–7), 469–477.