The Importance of a Fruit Juice HACCP Plan in Juice Production
Ensuring the quality and safety of beverages and fruit juices is a top priority for producers, given the risks and hazards associated with contamination. A comprehensive fruit juice Hazard Analysis Critical Control Point (HACCP) plan is essential for identifying and controlling hazards that could compromise product safety. This blog explores the critical role of a fruit juice HACCP plan, highlights key pathogens of concern, and emphasizes the importance of aseptic sampling in maintaining high standards in juice production.
Why a Fruit Juice HACCP Plan is Essential to Quality and Safety
A HACCP plan is more than just a regulatory requirement—it is a strategic framework that helps juice producers manage food safety risks. By systematically identifying potential hazards, determining critical control points (CCPs), and validating control measures, HACCP plans identify issues before they become problematic. This proactive approach is vital in an industry where consumer safety, trust, and product integrity are paramount.
Common Hazards in Juice Production
The most common hazards in juice production include mycotoxins, microbial, physical, and chemical contaminants:
- Mycotoxins (Patulin): Produced by fungi like Penicillium expansum, patulin can contaminate fruits and juice products, posing significant health risks. Monitoring for mycotoxins is essential to prevent contamination, especially in juices made from apples, mangoes, and oranges
- Microbial Contaminants: These are the most critical hazards, often involving pathogens like E. coli, Salmonella, and Cryptosporidium, which can survive in juice and cause severe foodborne illnesses. The presence of these pathogens is frequently linked to contaminated raw fruits, inadequate processing, or post-processing contamination. The high acidity of juices is not favorable to bacterial growth and has been considered a less risky environment. However, pathogens that can survive in juices still present a significant safety risk. Additionally, post-pasteurization contamination from primarily yeast and molds causes spoilage, impacting shelf life and product quality.
- Physical Contaminants: Foreign objects like glass or metal fragments can enter the juice during production, highlighting the need for rigorous equipment maintenance and inspection.
- Chemical Contaminants: Pesticides, residues from cleaning agents, and other chemicals can be a concern if not carefully managed through sourcing and production practices.
Mycotoxins and the Threat of Patulin in Juice Production
When developing an HACCP plan for juice manufacturing, it is essential to consider the potential hazards posed by mycotoxins, such as patulin. Patulin is produced by fungi such as Penicillium expansum, which can contaminate fruits and their derived products. A study conducted in Pakistan revealed that over 50% of mango and orange juice samples were contaminated with patulin, with some mango juices exceeding the regulatory limit of 50 µg/kg established by Codex Alimentarius and the European Union (Shabbir Hussain et al., 2020). These findings underscore the critical need for effective preventive measures and monitoring, such as Process Analytical Technology (PAT), to prevent fungal growth and reduce patulin contamination during processing, storage, and transportation. A robust juice HACCP plan should account for mycotoxin hazards and ensure compliance with regulatory standards to protect consumer health.
Food and Drug Administration’s Juice HACCP Guidance for Microbial Control
The Food and Drug Administration’s (FDA’s) juice HACCP regulation requires producers to establish control measures that achieve at least a 5-log reduction in the most resistant pathogens of concern. This standard applies to all juice processors except retail establishments that sell directly to consumers. Compliance with the FDA juice HACCP regulations significantly reduces the risk of outbreaks and ensures that the final product is safe for consumption (Source: FDA).
Centers for Disease Control and Prevention Validates Effectiveness of HACCP
Illness outbreaks from contaminated juice led to the creation of strict regulations like the juice HACCP rule, which mandates key control measures to reduce pathogen risks. Between 1995 and 2005, 21 juice-related outbreaks were reported, with apple and orange juice being the primary culprits, causing over 1,300 illnesses. Common pathogens included Salmonella, E. coli O157, and Cryptosporidium. Since the implementation of the juice HACCP regulation, juice-related outbreaks have decreased, although some operations that are exempt from the rule or non-compliant still pose risks. (Vojdani et al., 2008)
Suggested Sampling Points for Microbial Contaminants
A key component of a HACCP plan is identifying where sampling for microbial contaminants should occur. Recommended CCPs include:
- Raw Material Reception: Sample incoming fruits to assess the initial microbial load and detect potential contaminants before processing.
- Processing Equipment: Conduct routine sampling of equipment surfaces, including conveyors and cutting tools, to prevent cross-contamination.
- Post-Pasteurization Monitoring: Verify the effectiveness of pasteurization in reducing pathogen levels by sampling juice immediately after this step.
- Filling and Packaging: Monitor the packaging environment to ensure aseptic conditions are maintained, preventing recontamination during the final stages of production.
- Storage: Regular sampling during storage ensures that microbial growth is controlled, particularly in refrigerated environments.
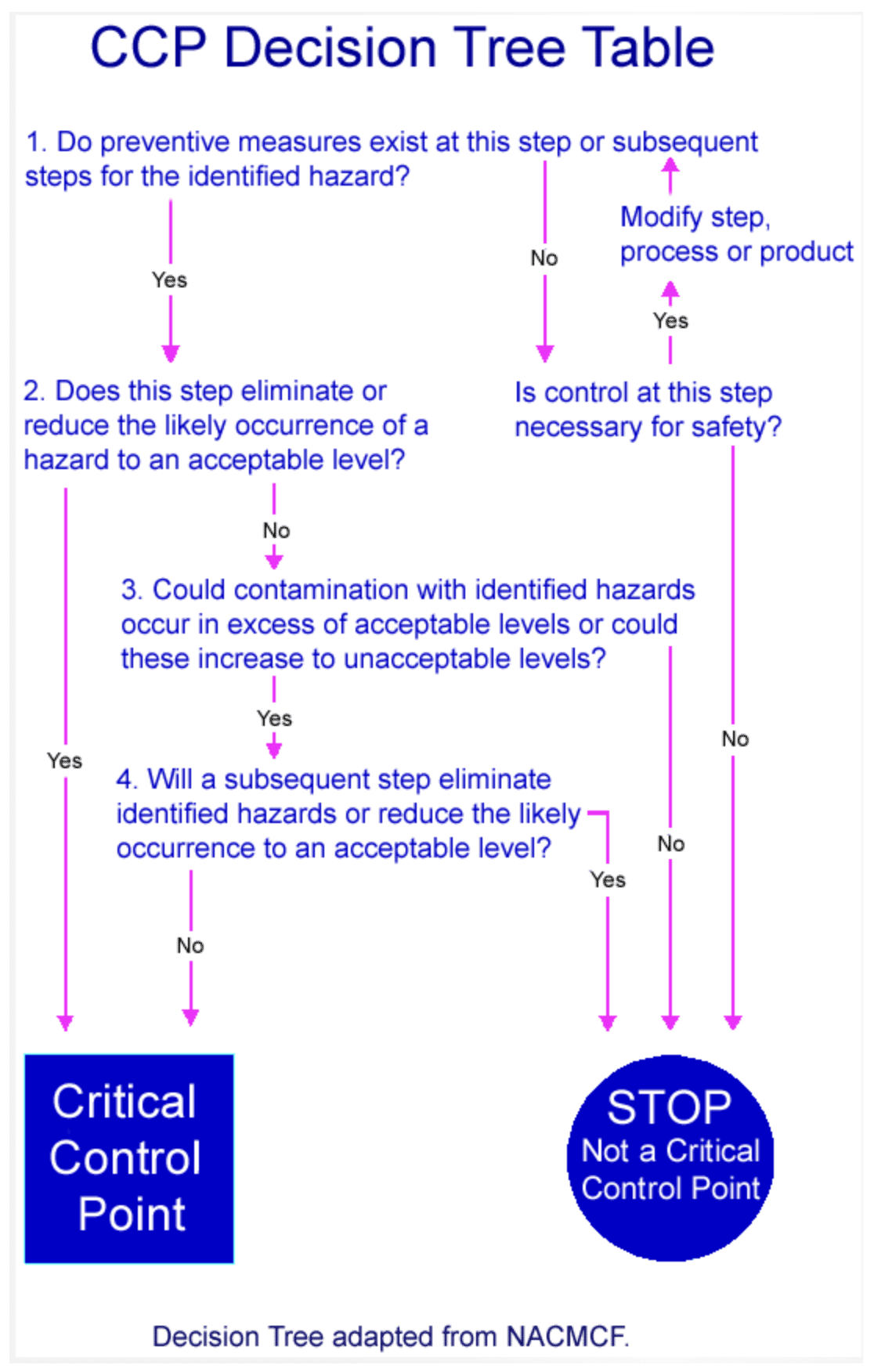
Aseptic Sampling and Process Analytical Technology in Juice Production
Aseptic sampling plays a crucial role in accurately assessing the microbiological safety of juice products. This method ensures that samples remain uncontaminated during testing, providing reliable data on juice quality. When combined with Process Analytical Technology (PAT), which offers real-time monitoring during production, the system helps detect and control microbial contamination at key stages like extraction and packaging. Inline process monitoring further enhances safety by allowing immediate adjustments, preventing contamination, and ensuring regulatory compliance throughout the juice production process.
Preventing Contamination through Proactive Measures
Proactive measures, such as preventive and predictive maintenance, are critical in preventing contamination during juice production. Regular equipment inspections, timely repairs, and consistent sanitation protocols help maintain control over potential hazards, minimizing the risks of microbial contamination. Predictive maintenance goes a step further by using data and monitoring tools to anticipate issues before they occur, preventing breakdowns and ensuring equipment is always functioning optimally. This proactive approach not only aligns with HACCP principles but also enhances overall operational efficiency, ultimately protecting juice quality and safety.
Invest in Quality Control for Long-term Success and Consumer Trust
A well-implemented fruit juice HACCP plan is a cornerstone of food safety in the juice industry. By adhering to FDA guidelines, identifying CCPs, integrating PAT for real-time monitoring, and employing aseptic sampling techniques, juice producers can effectively manage microbial risks. These proactive strategies help ensure product safety and regulatory compliance, safeguarding production quality and protecting brand reputation.
Have questions about aseptic sampling in juice production?
References:
Dewanti-Hariyadi, R. (2024). Microbiological Quality and Safety of Fruit Juices. FoodReview International.
Shabbir Hussain et al. (2020). Patulin Mycotoxin in Mango and Orange Fruits, Juices, Pulps, and Jams Marketed in Pakistan. Toxins. Retrieved from https://www.mdpi.com/2072-6651/12/1/52.
U.S. Food and Drug Administration. (2004). Guidance for Industry: Juice Hazard Analysis Critical Control Point Hazards and Controls Guidance, First Edition. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-juice-hazard-analysis-critical-control-point-hazards-and-controls-guidance-first.
Vojdani, J. D., Beuchat, L. R., & Tauxe, R. V. (2008). Juice-Associated Outbreaks of Human Illness in the United States, 1995 through 2005. Journal of Food Protection, 71(2), 356-364.




