Bacteriophage Control Using Closed Bulk Starter Vessel Inoculation – Part 2
Bacteriophage control and management for preventing phage invasions are challenges many dairy processing plants face. Starter culture bacteria play an essential role by converting milk sugar (lactose) into lactic acid in making dairy products such as cheese and yogurt.
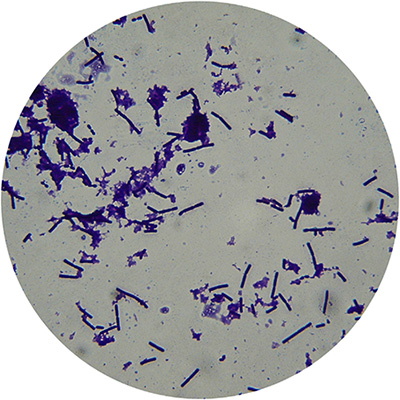
Photomicrograph of yogurt showing S. thermophilus and L. bulgaricus. (Image: Courtesy of Mullan, Michael. (2014).
Starter Cultures: Importance of Selected Genera. 10.1016/B978-0-12-384730-0.00321-9.)
Approaches to help prevent bacteriophage invasions
Quality management for bacteriophage requires a multifaceted approach and due diligence. Prevention, detection, and control are critical for avoiding the detrimental impacts of potential bacteriophage contamination on the quality of cultured dairy products.
To learn more about the potential impact of bacteriophage on starter cultures, read our recent blog, Proactive Bacteriophage Management – Part 1.
One of the primary methods for controlling bacteriophages for plants utilizing fermentation is to rotate the cultures used to ferment their product. This is effective but does not guarantee that there will not be bacteriophages for the rotating culture present in the plant. Therefore, an additional proactive line of defense is to reduce the fermentation vessel’s exposure to the environment.
Bacteriophage Control in Cheese Manufacture from Dairy Science Food Technology’s website provides historical background and a comprehensive listing and explanation of modern methods for controlling phage.
“The basic principles of phage control in commercial plants have been known since the early 1940s and the pioneering work of Dr. Hugh Whitehead and his colleagues in New Zealand.”
Source: Dairy Science and Food Technology
QualiTru’s system is proven effective for inoculation
One preventative method cited in the above article is an aseptic technique for inoculation of laboratory and intermediate cultures to prevent bacteriophage contamination. The process uses syringes for transferring cultures through a closed rubber stopper to ensure aseptic inoculation of bulk starter vessels.
A study was conducted at the University of Minnesota using the QualiTru (QMI) sampling system, Journal of Food Protection, Vol. 52, No. 10, (October 1989). Starter culture was aseptically injected through an inoculation port using a sterile needle and syringe in the presence of aerosol.
The study reached the following conclusion: While phage contamination may originate from sources other than aerosols in cheese factories, this inoculation system in which the starter medium was not exposed to aerosols reduced the risk of growing a contaminated bulk starter. Conversely, phage contamination and E. coli contamination were detected only from inoculated open vessel samples.
“Starter culture was aseptically injected through the inoculation port using a sterile needle and syringe in the presence of the aerosol. After 16 h of growth, the bulk starter was not contaminated with phage or E. coli.”
QualiTru’s system is proven to be effective for inoculation in closed bulk starter vessels or other fermentation vessels.
Installing a sampling/inoculation port in a fermentation vessel allows the technician to take samples and inoculate the product while minimizing the chance of introducing bacteriophage naturally present in the environment.




