Sterile or Aseptic? Understanding the Difference
Ensuring the quality and safety of dairy and food products is the number one priority of processors, explaining why questions surrounding sterile or aseptic frequently enter into the conversation, especially when discussing equipment cleanliness and sampling or testing techniques. While the words sterile and aseptic are often used interchangeably, they are not, in fact, interchangeable. Understanding the difference is important to ensure favorable outcomes in dairy and food processing, sample collection, and laboratory testing.
What Does Sterile Mean?

In general, sterile applies to equipment and environments. It is the concept of having or using equipment that is totally germ-free. To reach this level of cleanliness, equipment must be cleaned and sanitized using specialized procedures, sterilized using validated techniques in which the ability to kill all living organisms has been tested and verified, supplied in special packaging designed to ensure maintained sterility during storage and shipping, and handled in special ways.
In medicine, for example, sterile equipment and supplies are vital to prevent contamination and infection during surgery. Similarly, sterile needles are required so patients are not infected from inoculations or during a blood draw. While the circumstances are, of course, different, the concept is the same in dairy and food processing.
When sampling and testing equipment are introduced to a process and come into contact with food, they should be sterile so there is no danger of introducing harmful bacteria or other microbes. This attention to sterility plays a critical role in upholding the quality, shelf stability, and safety of dairy and other food products and is vital for making sure samples collected from the product are free from exogenous contamination.
What Is Aseptic?

This sounds remarkably close to the definition of sterile, but there is a subtle and highly important difference. Aseptic is a term that is generally applied to techniques that keep equipment or an environment sterile. Aseptic techniques are designed to prevent the introduction or transfer of microorganisms to an environment. In other words, they keep sterile things sterile.
In a dairy or liquid food processing environment, aseptic sampling technique is critical to control contamination of either the collected samples or the food product being sampled. Aseptic techniques are required for inserting sterile sampling equipment into the production line and for collecting samples through sterile equipment. If aseptic technique is not followed, the positive effect of sterile sampling equipment is lost, collected samples may be inaccurate or become contaminated, test results will be untrustworthy, and harmful organisms may be added to products being sampled.
Aseptic Sampling Is Critical to Testing Accuracy
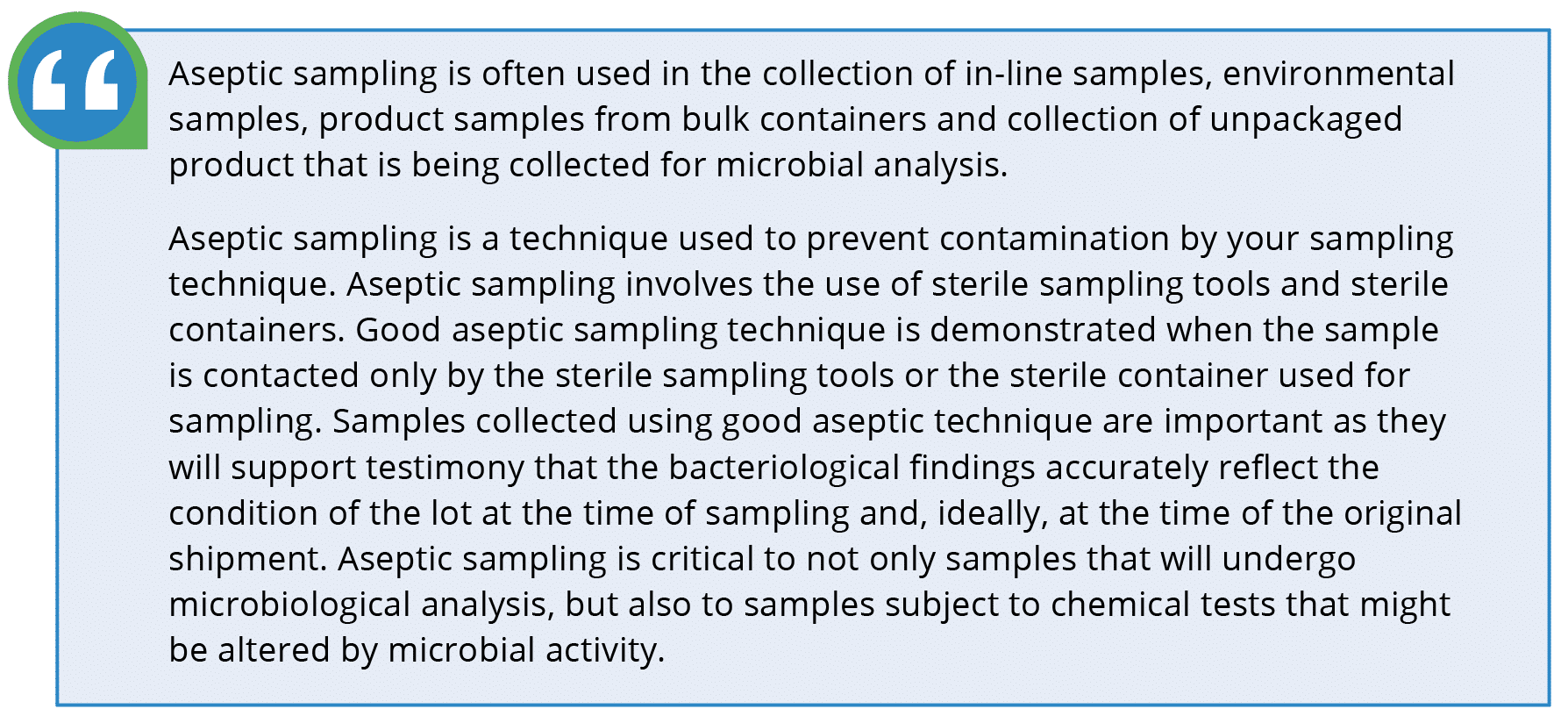
Dairy, brewery, beverages and liquid foods, biofuels, enzyme manufacturing, and nutraceutical and functional foods industries are vulnerable to a multitude of microbial contaminants that can compromise the quality and safety of their products. As such, the importance of proper sampling technique cannot be overemphasized. Aseptic sampling through sterile sampling equipment is critical to ensure food safety, product quality, and informed decision-making. Here’s what the U.S. Food and Drug Administration (FDA) says about aseptic sampling:
QualiTru® Sampling Systems has proudly manufactured sterile sampling equipment for thethe dairy, brewery, beverages and liquid foods, biofuels, enzyme manufacturing, and nutraceutical and functional foods industries for more than forty years and we have developed aseptic sampling protocols that have been proven by decades of use. Our products and processes have been accepted by the FDA and meet the agency’s rigorous standards for aseptic sample collection. QualiTru’s standard operating procedures (SOPs) for aseptic sample collection techniques are available here.
Is it Sterile or Aseptic?
Sterile means a device is “free from bacteria or other living organisms” and aseptic techniques are those that are designed to reduce the risk of introducing harmful or contaminating organisms to sterile equipment or into a sterile environment. They keep sterile things sterile. Whether sterile or aseptic, both are required to ensure the accuracy of samples collected for microbial tests, or those for chemical tests where the results could be impacted by microbial contamination. In the absence of sterile equipment and aseptic sampling techniques, the accuracy of collected samples cannot be assured and test results stemming from the samples cannot be relied upon.
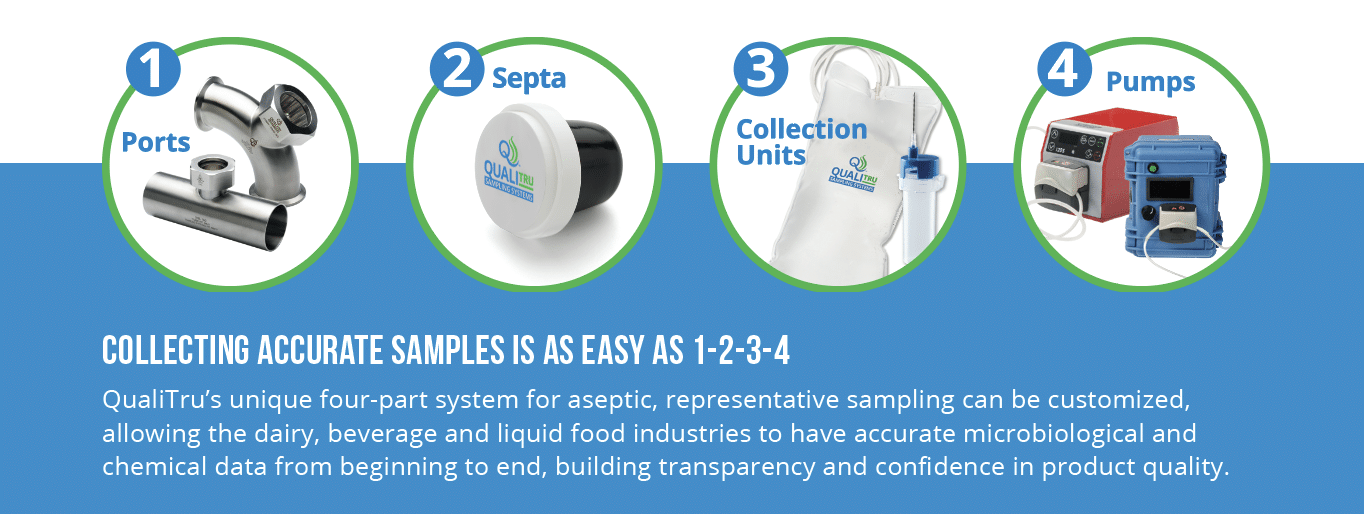
QualiTru delivers sterile products that meet the same rigorous sterility standards as those of the most delicate medical instruments. Our TruStream™ Septa, TruStream and TruMotion™ Collection Bags, and TruDraw® Sterile Single Use Samplers are sterilized using verified and validated procedures equal to those of medical devices. Following these high quality standards means our products meet the topmost levels of cleanliness and safety.
Installing the TruStream Septa using aseptic technique means samples can be collected immediately, without the need for additional sterilization steps, such as steam sterilizing. This means faster sample collection and lower labor costs, while eliminating the safety hazard of working with live steam and steam-heated equipment.
QualiTru’s High Quality Standards Mean You Can Trust Your Test Results
QualiTru’s high quality standards mean you can have confidence that the results coming out of your lab are accurate. Our sterile TruStream Septa, TruStream and TruMotion Collection Bags, and TruDraw Sterile Single Use Samplers, combined with aseptic sampling technique, make the sampling process accurate, reliable, and safe.
Resources
Articles and White Papers
Validation and Case Studies
Training Videos
FAQs
Have questions about aseptic and representative sampling? Ask Our Experts