Microbiological Control in NoLo Beer Production for Quality Assurance
The production of no and low-alcohol (NoLo) beers has gained significant traction as consumers increasingly seek healthier, lifestyle-friendly beverage options. In the evolving world of NoLo beer production, maintaining strict microbiological control is essential to ensuring quality, consistency, and safety. However, this segment presents unique microbiological challenges compared to traditional beers. The absence of alcohol, higher residual sugar levels, and reduced hop bitterness significantly elevate the risk of microbial contamination.
To meet these challenges, breweries must implement advanced microbial control measures tailored to the specific vulnerabilities of NoLo beer production. This blog explores the microbial risks and critical control points (CCPs) unique to this segment, highlighting how real-time monitoring solutions, such as the QualiTru TruStream™ sampling system, enable breweries to maintain high standards of quality, safety, and consistency through proactive quality assurance (QA).
Key Microbial Risks in NoLo Beer Production
Unlike traditional beers, NoLo beers lack the natural microbial barriers provided by alcohol and hop-derived compounds.
Alcohol inhibits spoilage organisms and pathogens, while hop-derived iso-α-acids limit bacterial growth. In NoLo beers, the absence or significant reduction of these compounds results in a higher vulnerability to microbial spoilage by organisms such as Lactobacillus brevis, Pediococcus spp., wild yeasts, and molds (Riedl et al., 2019; Schneiderbanger et al., 2018).
Due to incomplete fermentation, NoLo beers often contain higher residual sugars. These sugars serve as an ideal nutrient source for microbial proliferation, further exacerbating contamination risks. For example, Lactobacillus brevis thrives in environments with residual sugars and forms biofilms that are resistant to standard cleaning protocols.
The reduced hop bitterness and absence of alcohol in NoLo beers amplify risks during every stage of production. While there are no documented cases of foodborne pathogens in NoLo beers, the theoretical risks necessitate robust preventive measures. Effective microbial monitoring and stringent sanitation practices are critical to mitigating these risks (Riedl et al., 2019; Strantz et al., 1989).
Microbiological Critical Control Points in the Brewing Process
Effective microbial control in NoLo beer production hinges on identifying and managing CCPs throughout the brewing process. By focusing on these key areas, brewers can isolate potential contamination risks early, implement corrective actions, and ensure consistent, high-quality production. Below are the essential microbiological CCPs that every brewery should monitor.
Plate Heat Exchangers
Plate heat exchangers (PHEs) are critical for cooling wort efficiently. However, they also represent a significant contamination risk. Improper pressure differentials—where the coolant pressure exceeds that of the wort—can lead to microbial contamination via leaks. As part of a study investigating the survival of Salmonella typhimurium in dairy plant glycol and glycol/water cooling mixture, Zottola and Smith (1986) conducted a detailed survey of the pressure relationships in the cooling sections of eight High-Temperature Short-Time (HTST) pasteurizers. The results showed that 37.5% had higher pressures on the coolant side than on the pasteurized side. This pressure relationship could result in contamination of the product stream from the cooling medium. While this study focused on dairy processing (a highly regulated industry) and not NoLo beer, it points out a potential quality breach demanding continual monitoring.
While Salmonella typhimurium was the organism of focus in their study, its ability to persist in glycol/water mixtures highlights broader risks applicable to brewing. Beer spoilage bacteria, such as Lactobacillus brevis and Pediococcus damnosus, thrive in nutrient-rich environments and can form biofilms on PHE surfaces. Pinholes or cracks in the heat exchanger plates allow for leakage and create niches for biofilm formation, exacerbating contamination risks. Biofilms act as reservoirs for spoilage organisms, protecting them from cleaning agents and enabling continuous contamination of the product stream.
To address PHE vulnerabilities, breweries must implement continuous pressure monitoring, conduct regular inspections for mechanical integrity, validate cleaning protocols, and utilize product sampling upstream and downstream of a PHE to determine if it contributes to product contamination. These practices, combined with real-time microbial monitoring, help to pinpoint contamination sources that can then be promptly addressed to safeguard product quality (Zottola & Smith, 1986; Wagner et al., 2021).
Water Quality
Water is a primary ingredient in beer production and CCP due to its direct impact on product safety and quality. According to Strantz et al. (1989), inadequately treated water can introduce spoilage organisms such as Pseudomonas, Micrococcus, and coliform bacteria. These microbes thrive in water systems where organic nutrients are present, and their presence in finished beers can lead to off-flavors, haziness, and reduced shelf life.
Additionally, metals like iron, manganese, and zinc, which may leach into water from processing equipment or plumbing, can disrupt yeast metabolism and fermentation performance. Strantz et al. further noted that stagnant water or poorly maintained water systems can serve as reservoirs for biofilm formation, exacerbating contamination risks. Biofilms in water systems can be a persistent source of spoilage organisms, making regular maintenance and monitoring essential.
To mitigate water quality risks, breweries should employ robust water treatment systems, including reverse osmosis or ultraviolet (UV) disinfection, to ensure consistent water quality. Real-time microbial and chemical testing using QualiTru sampling systems can validate water purity and contamination loads throughout production before they impact the brewing process.
Post-Fermentation Transfers and Additions
Post-fermentation processes, such as dry hopping, fruit infusions, or the movement of beer through product transfer lines, represent critical points for microbial contamination. Wagner et al. (2021) determined that these activities create opportunities for spoilage organisms like Lactobacillus brevis and Pediococcus damnosus to contaminate the product. Ingredients such as fruits or hops are often contaminated with organisms that flourish in the nutrient-rich, no or low-alcohol environment of NoLo beers. Moreover, inadequately cleaned transfer lines, hoses, and vessels exacerbate the risk by serving as reservoirs for spoilage bacteria and biofilm formation.
To mitigate post-fermentation risks, breweries should prioritize stringent sanitation of all post-fermentation equipment, conduct microbial testing along transfer lines, and utilize sampling tools such as QualiTru sampling systems to monitor and isolate contamination hotspots.
Filling and Packaging Lines
The packaging stage is another CCP in NoLo beer production, as it represents a convergence of risks associated with biofilm formation and microbial contamination. Wagner et al. (2021) conducted an in-depth study examining microbial contamination and biofilm persistence across 23 sampling sites in a beer can filling line. They identified struts below the filler as a critical biofilm hotspot, harboring high concentrations of bacteria, yeast, extracellular DNA (eDNA), carbohydrates, and proteins. Despite cleaning efforts using advanced Cleaning-in-Place (CIP) systems, microbial residues remained on four sites post-cleaning, emphasizing the inadequacy of automated cleaning alone.
Additionally, hidden areas like filling heads, seals, and gaskets were found to accumulate biofilms, which, if not properly sanitized or replaced regularly, can contribute to contamination of finished products. The study highlighted the importance of targeted manual cleaning protocols in conjunction with automated systems to mitigate these risks effectively.
Complementing these findings, Schneiderbanger et al. (2018) analyzed 13,802 brewery samples over seven years, uncovering critical contamination patterns at packaging and late-stage production. Their work demonstrated that spoilage bacteria, such as Lactobacillus brevis and Pediococcus damnosus, were frequently detected in packaged products and filling lines. This underscores the need for real-time microbial monitoring to promptly identify and address contamination sources, preventing spoilage and ensuring product integrity.
To effectively address contamination risks in filling and packaging lines, breweries should adopt enhanced cleaning protocols that supplement automated CIP systems with targeted manual cleaning for persistent contamination sites. Biofilm monitoring is essential to identify high-risk areas, such as structural supports under filling equipment, where microbial residues often persist. In addition, real-time testing using tools like QualiTru sampling systems enables breweries to validate cleaning effectiveness and quickly detect residual contamination, ensuring ongoing product integrity and quality.
NoLo Beer Production Sampling Site Recommendations
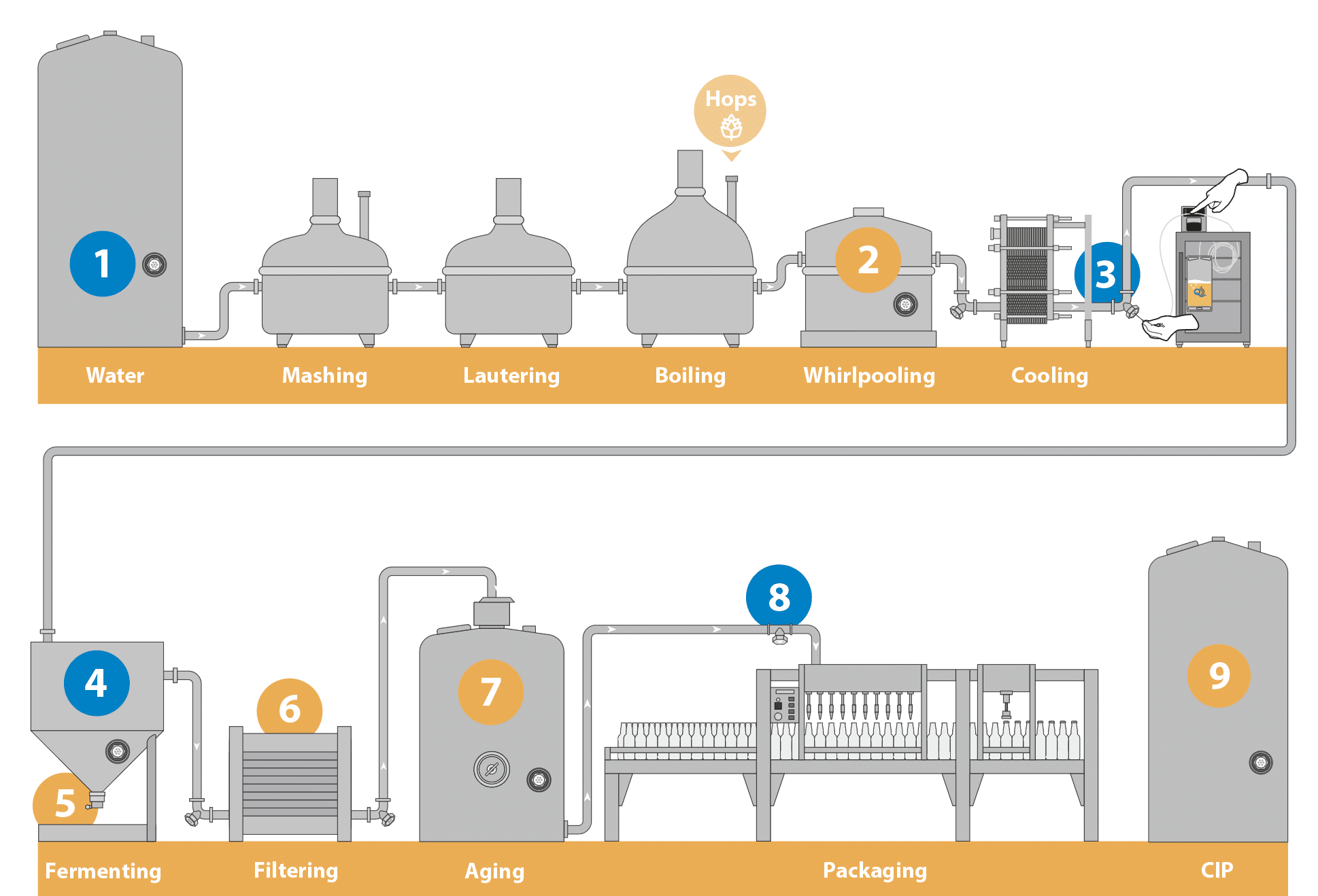
Gold (important): troubleshooting potential sources of contamination.
Benefits of Proactive Quality Assurance
- Early Detection: Continuous monitoring tools like QualiTru’s sampling systems allow breweries to identify contamination at its source before it escalates. For example, Wagner et al. (2021) demonstrated how microbial hotspots in filling lines, such as structural supports and gaskets, can be identified and addressed early through real-time microbial testing. This proactive approach prevents biofilms from forming and limits the spread of spoilage organisms.
- Cost Reduction: Preventing spoilage preserves product quality and reduces waste and costly recalls. Schneiderbanger et al. (2018) highlighted how breweries with robust QA measures, including regular microbial testing, experienced fewer contamination-related disruptions. Avoiding production halts and recalls significantly improves the bottom line.
- Data-Driven Decisions: Real-time data collection enables breweries to adjust processes, improving efficiency and product consistency. QualiTru sampling systems provide actionable insights by tracking contamination trends at CCPs such as plate heat exchangers, fermentation vessels, transfer lines, and fillers. This data-driven approach ensures timely and effective adjustments, reducing variability in the final product while enhancing overall operational control.
The Role of QualiTru Sampling Systems in NoLo Beer Sampling
QualiTru sampling systems are vital to ensuring microbial quality in NoLo beer production. The systems are designed to provide breweries with reliable, real-time insights into their production processes, enabling proactive quality assurance. Here are the key benefits:
- Real-Time Microbial Monitoring: QualiTru devices allow breweries to identify contamination issues as they arise, ensuring swift corrective actions to prevent spoilage and maintain product safety.
- Critical Control Point Verification: By integrating QualiTru sampling systems at key points like plate heat exchangers, fermentation or product holding vessels, water systems, and filling or transfer lines, breweries can validate cleaning efficacy and detect microbial hotspots.
- Process Efficiency: QualiTru TruStream Sampling Ports provide seamless access to production streams without interrupting processes, allowing frequent and sterile sample collection to optimize microbial testing protocols.
- Enhanced Traceability: Data collected through QualiTru TruStream Septa helps ensure sterile sample collection and accurate and representative product samples. Reliable data helps breweries pinpoint contamination sources and implement targeted interventions, reducing the likelihood of quality failures.
- Product Standards Compliance: QualiTru sampling systems help breweries meet stringent industry and customer standards by providing accurate and consistent microbiological testing data.
Elevating Quality Standards in NoLo Beer Production with Proactive Microbial Control
In the competitive NoLo beer market, maintaining microbiological quality is essential to protecting brand reputation, reducing waste, lowering costs, and ensuring consumer safety. By adopting advanced tools like QualiTru’s sampling systems and emphasizing proactive QA strategies, breweries can effectively mitigate microbial risks, improve operational efficiency, and deliver consistently high-quality products.
Invest in real-time monitoring solutions today to safeguard the future of your NoLo beer production. Contact QualiTru at (651) 501-2337 or email [email protected] for expert guidance and solutions tailored to your brewery’s needs.
References:
Zottola, EA, LB Smith. Survival of Salmonellae in refrigerated agitated water and water/glycol mixtures. J Food Protect, 1986; 49(6):467-470.
Riedl R, N Dunzer, M Michel, F Jacob, M Hutzler. Beer enemy number one: Genetic diversity, physiology, and biofilm formation of Lactobacillus brevis. J Inst Brewing, 2019; 125:250-260.
Wagner EM, S Thalguter, M Wagner, K Rychli. Presence of microbial contamination and biofilms at a beer can filling production line. J Food Protect, 2021; 84(5):896-902.
Strantz AA, EA Zottola, RL Petran, BJ Overdahl, LB Smith, L. B. The microbiology of sweet water and glycol cooling systems used in HTST pasteurizers in fluid milk processing plants in the United States. J Food Protect, 1989; 52(11):799-804.
Schneiderbanger J, M Grammer, F Jacob, M Hutzler. (2018). Statistical evaluation of beer spoilage bacteria by real-time PCR analyses from 2010 to 2016. J Inst Brewing, 2018; 124:173-181.




