The Dirty Truth: How Biofilms in Milking Equipment May Fuel Mastitis in Dairy Cows and Threaten Dairy Hygiene
Biofilms, resilient bacterial communities encased in a self-produced matrix, pose a significant threat to dairy farming. These microbial formations develop on surfaces that are frequently exposed to moisture and organic material, making milking equipment an ideal breeding ground. Once established, biofilms are notoriously difficult to remove, resisting standard cleaning protocols and allowing pathogenic bacteria to persist. The presence of biofilms in milking equipment has been linked to outbreaks of mastitis in dairy cows, contaminating milk and contributing to persistent infections within dairy herds (Vargová et al., 2023). This article explores how biofilms in dairy equipment act as reservoirs for mastitis-causing bacteria, the implications for udder health, and strategies for early detection and prevention.
Biofilms in Milking Equipment: A Hidden Risk to Dairy Hygiene
Biofilms form when bacteria adhere to a surface and begin producing extracellular polymeric substances (EPS) that create a protective barrier. This structure shields the bacteria from disinfectants and mechanical cleaning, making biofilms particularly challenging to eliminate. In the dairy industry, biofilms accumulate in critical areas such as milking hoses, teat cups, and bulk tanks, where they serve as reservoirs for pathogenic bacteria like Staphylococcus aureus and Streptococcus uberis (Medina et al., 2025).
The conditions within milking equipment favor biofilm development. Nutrient-rich milk residues provide ample resources for microbial growth, while rubber and plastic surfaces offer a textured substrate for bacterial adhesion. Medina et al. (2025) found that rubber components, including Buna-N and EPDM, are particularly susceptible to biofilm formation, which increases the risk of persistent contamination. Without effective sanitation and equipment maintenance, these biofilms continuously shed bacteria into the milking system, contaminating milk and exposing cows to infection.
How Dirty Equipment in the Entire Milking System May Contribute to Mastitis in Dairy Cows
Mastitis, an inflammatory condition of the udder, remains one of the costliest diseases in the dairy industry. Biofilms play a crucial role in mastitis outbreaks by acting as persistent reservoirs of bacterial infection. Vargová et al. (2023) found that S. aureus isolated from milking equipment exhibited strong biofilm-forming capabilities, increasing its ability to persist in the dairy environment and cause infections.
Biofilms in milking equipment and throughout the milking system—including pipelines, milk meters, coolers, and bulk tanks—serve as persistent reservoirs of mastitis-causing bacteria, continuously shedding pathogens that expose the herd to infection. These biofilms thrive in areas where milk residues accumulate, creating ideal conditions where Staphylococcus aureus and Streptococcus uberis may persist despite cleaning efforts (Latorre et al., 2019). Even after sanitation cycles have been run, biofilms often remain in hard-to-reach areas, leading to repeated bacterial exposure and reinfection at the teat level. Bacteria from contaminated surfaces enter the teat canal during milking, contributing to intramammary infections and cross-infection. Additionally, biofilms in milk pipelines, cooling tanks, and bulk tanks can indirectly reintroduce bacteria onto the udder via vacuum fluctuations, milk mist, or incomplete cleaning (Medina et al., 2025). When milk from different sessions is combined in improperly cleaned bulk tanks, residual bacteria mix with fresh milk, prolonging bacterial persistence. This recurring contamination cycle, as described by Lee et al. (2014), underscores the difficulty of eradicating biofilm-associated mastitis pathogens from dairy environments. The failure to effectively control biofilms in milking systems not only leads to chronic mastitis but also results in economic losses due to increased veterinary costs, reduced milk production, and premature culling of affected animals.
The Role of Biofilms Inside the Udder: A Persistent Reservoir
While biofilms in milking equipment contribute to mastitis development, biofilm formation inside the mammary gland further complicates treatment. Kukhtyn et al. (2016) demonstrated that S. aureus forms biofilms within the udder, allowing infections to persist despite antibiotic therapy. These biofilms create a physical barrier that prevents antimicrobial agents from reaching the embedded bacteria, making mastitis difficult to cure.
In a similar study, Lee et al. (2014) found that S. aureus strains from subclinical mastitis cases exhibited increased resistance due to biofilm production. This resistance leads to chronic infections, where bacteria persist in the udder and intermittently shed into the milking system, creating a cycle of reinfection. The persistence of biofilms within the mammary gland means that cows with subclinical mastitis can continuously shed bacteria into the milking system, contaminating equipment and further exacerbating the problem. Unlike biofilms that originate in milking components and spread bacteria to cows, these internal reservoirs maintain an ongoing cycle of reinfection by introducing bacteria back into the milking system, where they can colonize milking equipment, spread to other cows, and persist in the herd. This contamination cycle differs from the direct introduction of bacteria from milking components, as it represents a continuous loop of pathogen persistence that is reinforced with each milking session.
Detecting and Eliminating Biofilms Throughout the Milking System
One of the biggest challenges in controlling biofilms is their ability to remain undetected until they cause significant contamination. Undetected biofilms in pipelines and bulk tanks may not only contribute to milk contamination but also serve as persistent sources of reinfection for cows, maintaining a cycle of contamination within the herd. While teat-end biofilms are a direct risk to udder health, biofilms in the wider milking system create a long-term bacterial reservoir that can lead to repeated exposure to mastitis-causing pathogens (Vargová et al., 2023). These biofilms not only contaminate milk but also lead to repeated exposure of cows to mastitis-causing pathogens. Studies suggest that when bacterial-laden milk mist backflows through the system, it can deposit pathogens onto teat surfaces, further increasing infection risk (Latorre et al., 2019).
Preventing Mastitis with Biofilm Control and In-Process Sampling
In-process sampling provides a proactive approach to biofilm management by identifying contamination points before they lead to widespread infection. By routinely sampling critical control points (CCPs) such as the receiver, balance tank, filters, chiller or milk cooler, milking hoses and pipelines, and farm silos or bulk tanks, farmers can detect biofilm hotspots early and implement targeted sanitation measures. In-process sampling allows dairy operators to identify bacterial persistence at various stages of the milking and milk storage process, ensuring that bacteria are not reintroduced into the system through hidden biofilms. This proactive approach helps eliminate biofilms before they mature and reduces the risk of mastitis outbreaks and milk contamination.
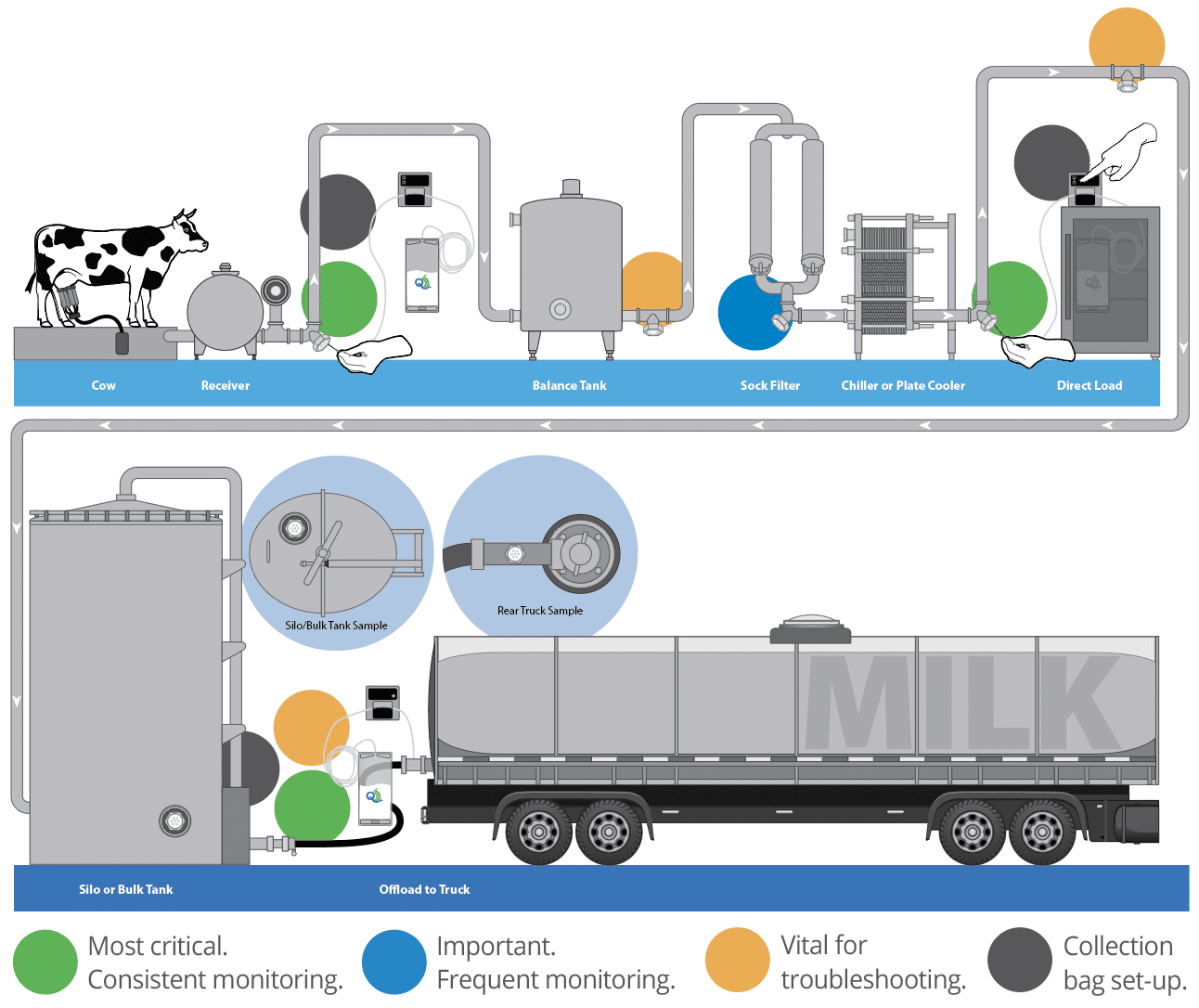
Most critical (Green): These are the most essential steps in the process that need consistent monitoring:
- Milk coming out of the receiver represents the commingled milk of the group of cows (string) currently being milked in the milking parlor. Samples should be collected at the receiver at least once for each string. The TruDraw® Sterile Single Sampler is ideal when a small sample is required. However, the best method to collect the sample consistently throughout milking is by using a TruMotion™ 2 liter Collection Bag and Watson-Marlow peristaltic pump (or equivalent QualiTru peristaltic pump offering).
- Milk from the plate cooler (chiller) is the next CCP. A sample should be collected immediately downstream of the plate cooler. This sample will indicate whether the equipment was properly cleaned and sanitized. If contamination increases from the receiver to this sampling point, the equipment is likely soiled, or biofilms may be present at some point in the process. This sample should be collected early in the milking of each string using a TruDraw sampling vial or equivalent.
- Milk coming out of the bulk tank or being loaded directly onto the milk tanker is the final step before the milk leaves the farm. The farm manager should want this sample collected to ensure the bulk tank is cleaned correctly and as evidence of the quality of the milk that is being released to the dairy processor. This sample should be collected over the entire course of loading using a TruMotion Collection Bag and peristaltic pump.
Important. (Blue): Frequently collecting a sample immediately upstream of the plate cooler is valuable. This is done for an important reason: to isolate the plate cooler. Occasionally, cracks or pinholes develop in cooler plates, or gaskets become worn and cracked. These defects can allow contaminants to enter the milk from the water or glycol coolant used to chill the milk.
Milk is cooled in the plate cooler as it runs over one side of a thin stainless steel plate with very cold chill-water or water plus glycol coolant running on the other side in counter-current flow. Theoretically, the pressure on the milk side of the plate is higher, so if a leak occurs, milk will flow in the direction of the coolant rather than the other way around. However, this pressure gradient is often improperly maintained due to operator inattention or a change in flow from a pump. Monitoring for this potentiality is valuable for troubleshooting but not critical daily.
A sample from this location should be taken using a TruDraw sampling vial or equivalent. The sample should be collected early in the milking of the first string, and results should be compared to the sample collected downstream of the chiller.
Vital for troubleshooting potential sources of contamination (Gold): Sampling locations at various points along the process should be established so equipment can be isolated. For example, a sampling port after the balance tank will isolate the process from the receiver through the balance tank. A sampling port before the bulk tank will isolate transfer lines from the chiller to the bulk tank, and so on. This sampling method is valuable in troubleshooting contamination problems by narrowing down where contamination might originate. It is tough to identify sources of contamination unless the process can be broken down into small segments.
Similarly, a sampling port in the door of the bulk tank (or silo) will isolate the bulk tank. Large vessels, such as bulk tanks or milk silos, are challenging to clean and are subject to failure in the stainless steel leading to cracks or pitting that can harbor bacterial contaminants. Biofilms readily attach to pitted or cracked stainless steel; once established, they are challenging to find and eradicate.
Maintaining herd health and milk quality starts with identifying risks at the source. QualiTru’s aseptic sampling systems provide a precise, dependable solution for monitoring contamination throughout the milking system. By enabling early detection of biofilms and bacterial threats, these systems support proactive mastitis prevention and help ensure dairy hygiene—from the parlor to the bulk tank.
- Inline Sampling
- TruStream7 Tri-Clamp Tee 2” (Part # 215147) or TruStream 7 Tri-Clamp Elbow 2″ (Part #213029)
- TruStream7 Septa (Part # 110011)
- TruStream 250ml/18g (Part # 111450) for a representative sample or the TruDraw® Sterile Single Sampler (Part #112021) for a small, aseptic sample
- Holding Tank Sampling
- TruStream7 Recessed 4”x 2” (Part # 212123)
- TruStream7 Septa (Part # 110011)
- TruDraw® Sterile Single Sampler (Part # 112021)
Breaking the Cycle: Controlling Biofilms Before They Harm the Herd
To effectively combat biofilms in milking equipment and prevent their impact on herd health, dairy farmers should adopt a combination of preventative strategies:
- Regular replacement of rubber components: Medina et al. (2025) found that older rubber parts provide an ideal surface for biofilm formation. Replacing liners, gaskets, and hoses at recommended intervals reduces contamination risk.
- Enhanced cleaning protocols: Standard alkaline and acidic cleaning solutions often fail to remove mature biofilms. Enzymatic treatments and mechanical scrubbing can improve cleaning efficacy.
- Routine in-process sampling: By detecting biofilms early, dairy producers can take corrective action before contamination spreads.
Biofilms in the milking system are a persistent yet often overlooked threat to dairy hygiene and herd health. They do not just exist at the teat cup level but can be found throughout the milking infrastructure, increasing the risk of continuous reinfection and cross-contamination. By acting as reservoirs for mastitis-causing bacteria, biofilms contribute to chronic infections, increased culling rates, and potential milk contamination. While pasteurization ensures the safety of commercial dairy products, the growing demand for raw milk and the cost of persistent mastitis outbreaks highlight the importance of stringent biofilm control measures.
In-process sampling offers a proactive solution for detecting and eliminating biofilms before they cause harm. Combined with improved sanitation practices and material management, these strategies can help break the cycle of biofilm-associated mastitis and contamination.
Proactively fight mastitis at its source by prioritizing biofilm control and in-process sampling. Improve dairy hygiene and safeguard your herd’s health with QualiTru’s aseptic sampling solutions. Contact us today!
Have questions about biofilms in milking equipment?
References:
Kukhtyn MD, VL Kovalenko, YV Horyuk. Bacterial biofilms formation of cattle mastitis pathogens. J Vet Med Biotech, 2016; 2(4):8-15.
Latorre AA, PA Pachá, G González-Rocha. On-farm surfaces in contact with milk: The role of Staphylococcus aureus-containing biofilms for udder health and milk quality. Foodborne Path Dis, 2019; 17(1):44-51.
Lee SHI, BLC Mangolin, JL Gonçalves, DV Neeff. Biofilm-producing ability of Staphylococcus aureus isolates from Brazilian dairy farms. J Dairy Sci, 2014; 97(7):4121-4130.
Medina C, D Manriquez, BA Gonzalez-Córdova, PA Pacha, JM Vidal, R Oliva, AA Latorre. Biofilm forming ability of Staphylococcus aureus on materials commonly found in milking equipment surfaces. J Dairy Sci, 2025. In Press.
Vargová M, F Zigo, J Výrostková, Z Farkašová. Biofilm-producing ability of Staphylococcus aureus obtained from surfaces and milk of mastitic cows. Vet Sci, 2023; 10(6):386.




