Mold Contamination in Juice Processing: Hidden Risks, Heat Resistance, and the Role of Aseptic Sampling
In juice processing, microbial contamination remains a leading cause of product spoilage and shelf life failure. Much of the scientific attention in juice quality has centered on Alicyclobacillus (ACB), a heat-resistant, acidophilic bacterium that produces off-odors reminiscent of medicinal or smoky taint. ACB is a legitimate concern, especially for shelf-stable juices subjected to pasteurization, but it is far from the only microbial threat. In fact, juice contamination by molds—often overlooked in regulatory discourse—represents a more pervasive and costly risk to juice quality, brand reputation, and consumer safety.
For that reason, this blog focuses on mold: how it enters and survives in juice processing environments, the conditions that promote its growth, the risks it poses to product quality, and the critical role of aseptic sampling in its detection and control. While ACB may dominate scholarly discussion, mold often proves the more stubborn and insidious spoiler in the practical realities of juice production.
Persistent Mold Contamination Risks in Juice Processing Facilities
Molds are filamentous fungi capable of thriving in environments traditionally thought to be hostile to microbial life. Many juice products are low in pH, high in sugars, and thermally treated—factors that inhibit most bacteria. Yet these are the same attributes that often create ideal growth conditions for mold, particularly those capable of surviving heat processing.
Some spoilage molds, such as Byssochlamys, produce heat-resistant ascospores that can survive pasteurization and subsequently germinate in packaged products (Beuchat, 1986). These organisms are not limited to raw materials but can colonize environmental niches within the facility, including poorly drained areas, improperly cleaned seals, or airborne pathways. Post-pasteurization contamination is of particular concern. Air handling systems, gaskets, transfer hoses, and filler heads all represent potential entry points for mold spores, especially when hygienic design or air filtration is suboptimal (Tournas, 2005).
Unlike bacterial contamination, mold growth may take weeks to become visible or produce spoilage markers. The problem often escapes detection during in-plant quality checks and only emerges after distribution, leading to consumer complaints, reputational damage, or costly recalls. Shelf-stable juice products are particularly vulnerable, as they provide molds with time and nutrients to proliferate undetected.
Conditions That Encourage Mold Growth and Juice Spoilage
Several conditions in juice processing environments contribute to mold establishment and persistence. Moisture accumulation—whether from condensate, leaks, or product spills—creates microenvironments ideal for mold colonization. Stagnant zones around seals, gaskets, and dead legs in piping are especially notorious for harboring contamination that survives even aggressive sanitation.
Frequent production changeovers or seasonal variation in raw materials may also introduce new sources of mold spores. Airborne transmission remains a constant threat; inadequately filtered or cleaned ventilation systems can transport spores from high-risk areas, such as fruit receiving bays, to downstream processing zones.
While mold may be introduced via raw materials, its survival and spread within processing environments are often linked to facility design and sanitation strategy. Research has shown that even under rigorous cleaning protocols, mold spores can persist in biofilms or protective niches on equipment surfaces (Silva, et al., 2018). This persistence underscores the need for environmental monitoring programs that extend beyond visual inspection or periodic swabbing.
Spoilage Risks from Juice Contamination by Molds and Quality Impacts
The consequences of mold contamination extend beyond aesthetics. Mold can lead to off-flavors, turbidity, sediment formation, and visible growth on packaging surfaces—all of which result in consumer complaints or product rejection. In some cases, gas production from mold metabolism can cause bloating or rupture of sealed containers, creating potential safety risks.
Mold spoilage also undermines shelf life claims. A juice product that leaves the plant in apparent compliance may degrade during transport or retail storage if mold was present at low but viable levels. Because mold effects are often delayed, processors face both the cost of post-market failures and the challenge of tracing contamination sources weeks after the fact.
Control Points for Mold Prevention
Mitigating mold contamination requires a comprehensive approach rooted in hygienic design, environmental controls, and routine microbial monitoring. Key control points include:
- Post-pasteurization zones, where the product is no longer protected by heat and is vulnerable to airborne or equipment-borne contamination.
- Tank lids, valve assemblies, and gaskets, which can harbor spores if not adequately cleaned, maintained, and routinely inspected.
- Air handling systems, which should maintain positive pressure, appropriate filtration (e.g., High-Efficiency Particulate Air (HEPA)), and a consistent maintenance schedule to reduce airborne spore load.
A robust sanitation program, while critical, is not sufficient on its own. Verification of cleaning effectiveness must be reinforced through routine microbial sampling, especially in areas that are inaccessible to surface swabbing or difficult to clean thoroughly with clean-in-place (CIP) systems.
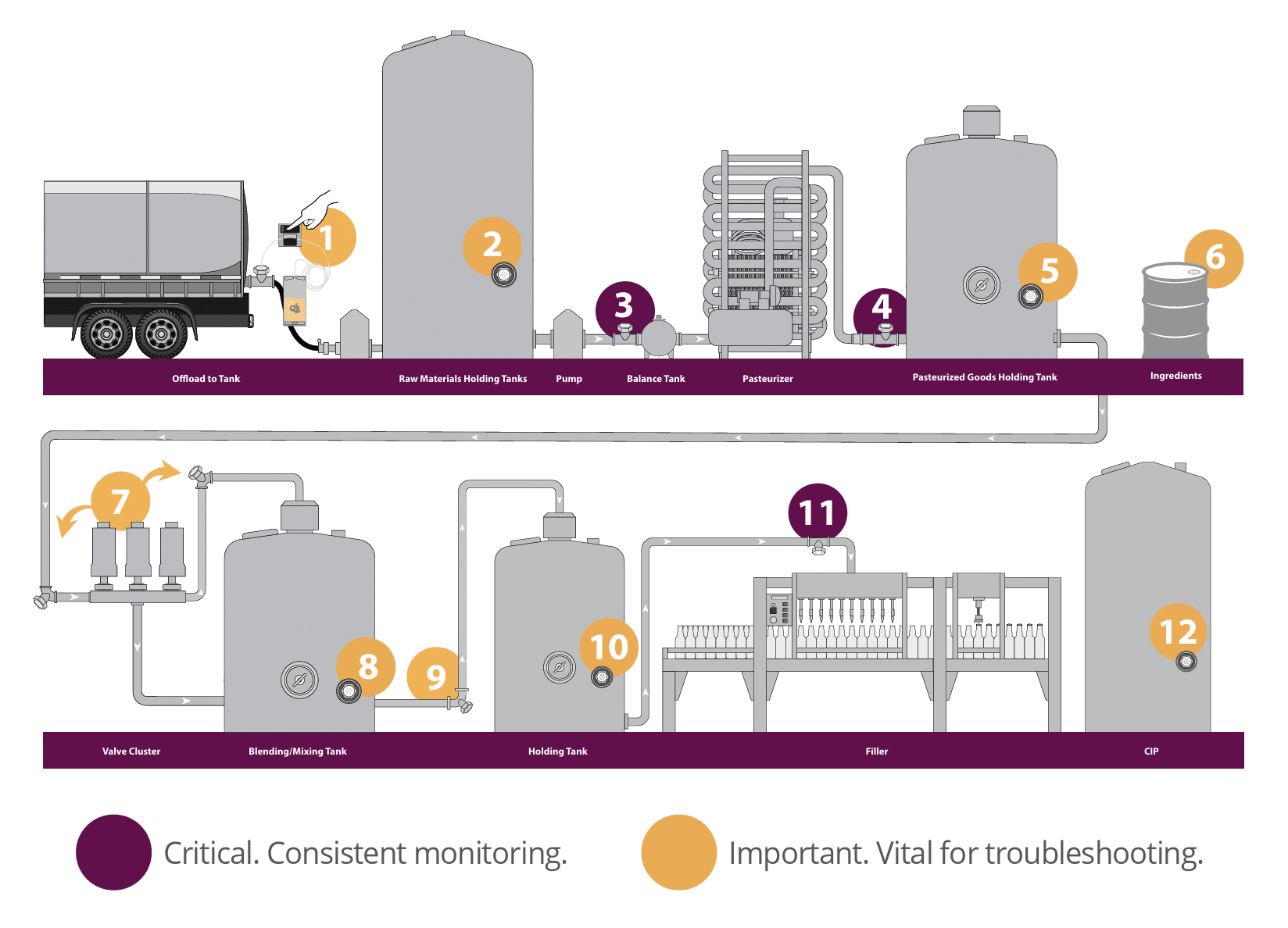
Most critical (Maroon): These are the most essential steps in the process that need consistent monitoring:
- Prior to Balance Tank: Install a QualiTru TruStream Sanitary Port immediately ahead of the pasteurizer’s balance tank and sample with a TruDraw collection vial. Test results on these samples are compared to those taken at the receiving bay and the HTST pasteurizer’s discharge to confirm the effectiveness of pre-pasteurization sanitation procedures and microbial reduction.
- Post-Pasteurization Monitoring: Install a QualiTru TruStream Sanitary Port on the discharge side of the pasteurizer to monitor for post-pasteurization contamination and validate pasteurization effectiveness. This application supports regulatory compliance, diagnostic assessments, system quality checks, CIP effectiveness verification, and overall microbial quality assurance.
- Filler: Install a QualiTru TruStream Sanitary Port immediately upstream of the filler to conduct regulatory and quality-system sampling. Samples collected here verify the sterility and absence of microbial contamination immediately before packaging, ensuring the final product’s safety and quality.
Vital for Troubleshooting Potential Sources of Juice Contamination (Gold): Sampling points highlighted in gold should be monitored when diagnosing sources of post-pasteurization contamination in juice processing. Ideally, install aseptic sample collection ports in each raw and pasteurized juice holding tank; before and after mixing or blending tanks; upstream and downstream of valve clusters; and immediately before and after additional critical processing equipment. Strategically positioned ports enable precise isolation of equipment segments, clearly identifying where contamination originates and facilitating effective corrective actions to ensure consistent juice product safety and quality.
Ensuring juice quality and safety requires precise microbial monitoring throughout the processing line. QualiTru’s aseptic sampling systems offer juice processors a dependable, contamination-free method to accurately detect mold and microbial contaminants. With consistent and representative sampling, QualiTru’s solutions empower proactive quality management, safeguarding your juice products, ensuring HACCP compliance, and protecting your brand’s reputation.
- Inline Sampling
- TruStream7 Tri-Clamp Tee 2” (Part #215147) or TruStream 7 Tr9-Clamp Elbow 2″ (Part #213029)
- TruStream7 Septa (Part # 110011)
- TruStream 250ml/18g (Part #111450) or TruMotion 2L/18g w/2.0mm Tubing (Part #111770) with a Watson-Marlow Pump (Part #500000) or QualiTru Peristaltic Portable Pump (Part #500200) for a representative sample or the TruDraw® Sterile Single Sampler (Part #112021) for a small, aseptic sample
- Holding Tank Sampling
- TruStream7 Recessed 4”x 2” (Part #212123)
- TruStream7 Septa (Part #110011)
- TruDraw® Sterile Single Sampler (Part #112021)
- Barrels, Drums, and Totes Sampling
- TruStream3 NPS Drum Sampling Cap (Part #241010)
- TruStream7 Septa (Part #110011)
- Syringe 60cc (100 per Case) (Part #113120)
Using Aseptic Sampling to Prevent Mold Contamination in Juice
A critical but sometimes underutilized tool in mold control is inline aseptic sampling. Unlike ad hoc or grab sampling, which are susceptible to contamination and inconsistencies, aseptic systems offer a closed, sterile pathway for collecting representative samples at critical points in the process.
QualiTru’s aseptic sampling systems are engineered to support microbial monitoring in high-risk processing environments. When installed at appropriate post-thermal or pre-packaging locations, these systems enable juice processors to:
- Detect heat-resistant molds that may survive pasteurization,
- Monitor microbial trends to identify early indicators of contamination,
- Validate the effectiveness of sanitation procedures, especially during changeovers or startups, and
- Support Hazard Analysis and Critical Control Points (HACCP) and Food Safety Modernization Act (FSMA) compliance by documenting preventive control verification.
These systems also integrate seamlessly into Environmental Monitoring Programs (EMPs), enabling consistent data collection for trending, root cause analysis, and continuous improvement.
Proactive Mold Management and Juice Shelf Life Optimization
As the juice and beverage industry continues to pursue extended shelf lives, global distribution, and higher quality standards, the importance of mold control will only increase. While Alicyclobacillus remains an important bacterial concern, mold often presents a more persistent, unpredictable, and visually disruptive threat. Its ability to survive processing, evade early detection, and exploit minor design flaws makes it a formidable spoilage agent.
A proactive mold control program, such as preventive and predictive maintenance, begins with understanding the unique risks within a facility and implementing targeted, validated cleaning protocols. But it must also include robust microbial monitoring—powered by tools like aseptic sampling systems that deliver reliable, actionable insights. With these capabilities in place, processors can shift from reactive problem-solving to preventive quality assurance, safeguarding both their products and their brands.
Have questions about aseptic sampling to prevent mold Contamination in juice?
References
1. Beuchat LR. Extremophilic fungi: The moldy menace of food processing. Food Technology, 1986; 40(11): 80–88.
2. Silva SC, JM Almeida, CF Silva. The ecology of spoilage molds in fruit juice processing: Environmental niches and survival mechanisms. Journal of Food Protection, 2018; 81(9): 1425–1432.
3. Tournas VH. Molds and yeasts in fresh and minimally processed vegetables, and sprouts. International Journal of Food Microbiology, 2005; 99(1): 71–77. https://doi.org/10.1016/j.ijfoodmicro.2004.08.022




