The SHACCP Explained
I trust we all know HACCP. HACCP and me go back a long way; as a matter of fact, we were conceived in the same year! When I came across HACCP during my student years, I thought it was really boring; HACCP being methodical, slow and elaborate and me being young and restless. However these days, HACCP has become a big part of my professional life and I have applied the HACCP principles in many different ways. This means I must have changed somewhat, because I don’t believe the HACCP principles have changed since their inception.
The basic premise of HACCP is this: if we have a process, we can map this process and ask ourselves at each process step: “how do we control this step and what can go wrong?”. Once we have identified those things that can go wrong (the “hazards”), we can start thinking about how these hazards could effect the outcome of our process (determine risk) and what additional controls we can introduce to strengthen the process.
HACCP is a risk-based, preventive tool and the idea of SHACCP is to apply this tool on our diagnostics process. To illustrate, lets map a typical process flow from taking the sample to the sample reception in the laboratory:
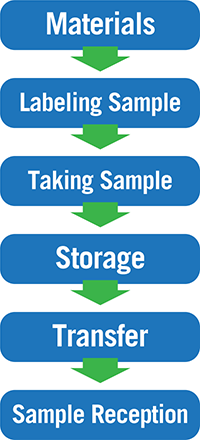
As you can see, we already have quite a few process steps, and we haven’t even included sub-sampling or the laboratory yet! Putting on our SHACCP hat, we can now start thinking about the hazards that may affect our sample integrity, and ultimately, our test result. Let’s throw in some risks I have seen during my career.
Materials
- Do we have the right sample container?
- Do we have the correct sample tool?
- Will the sample be representative?
- What are the potential risks of my sample tool?
- Have we got the right disinfectant?
Labeling Sample
- Do we have an exact labeling protocol?
- Do we pre-label or label Just-In-Time?
- Do we capture all the information?
Taking Sample
- Have we clearly defined our method?
- Is it an aseptic technique?
- Does the sampler disinfect their hands?
- Does the sampler operate the sampling equipment correctly?
- Do we have adequate training and clear procedures?
Storage
- Are samples stored in a controlled environment?
- Are samples stored in a clean environment?
- How long are samples stored?
And the list goes on!
As you can see, my preferred way to identify hazards is by asking questions. Ideally, we do this with a team, which should include a sampler and a laboratory technician, to get the most comprehensive list.
Finding a whole list of hazards may feel overwhelming. However, most items on the list will already be controlled in some form and not be a significant risk. For example, we may have a system with pre-labeled sample containers, which are issued each day, and the only hazard that remains is that the operator fills the right box at the right time.
So, how do we turn a hazard into a risk? Let’s look at the sample transfer time. If we ship our samples to an off-site laboratory, a danger is that we encounter delays as a result of traffic disruptions. To determine the risk of the transfer delay hazard on our sample, we can use likelihood versus impact.
For sensitive, non-stable food samples, the impact of a significant transfer delay may be microbiological growth or decline in the sample with the micro test result likely to be affected. The likelihood of the delay depends on where you are in the world; however, even in rural New Zealand, we have experienced some significant delays due to weather events. That means if we have a sensitive sample and a likelihood of transfer delays, the transfer delay hazard has turned into an actual risk for our sample integrity.
So, what can we do? How do we strengthen our controls of the diagnostic process transfer step and manage this transfer delay risk? One thought would be to instruct the laboratory monitor the “sample to receipt” time for our sensitive micro samples and not test if it exceeds X hours. This change in expectations is because the sample would no longer reflect the actual process conditions, and the test result may be false.
What happens if we decide to test the sample anyway? After all, it was chilled, right? Well, we may not only pay for an inaccurate test result but also run the risk of getting an unpleasant surprise. Particularly when it comes to food safety tests, you should never take the risk of testing a compromised sample, because you will need to act, even if you can subsequently point to a significant transfer delay!
Only if we submit accurate samples will we get value for money, gain confidence in the results, and become more accepting of the outcomes, even when they are unexpected.




