Maintaining the Quality of Pasteurized Fluid Milk
Maintaining the quality of pasteurized fluid milk is essential for consumer safety, shelf life, and product satisfaction. While pasteurization eliminates harmful pathogens, other factors, such as microbial contamination and post-pasteurization risks, can compromise milk quality. By implementing robust quality control measures, including regular sampling and testing, dairy plants can help ensure the safety and consistency of their fluid milk products.
Fluid milk, commonly known in the industry as milk processed for beverage use, continues to be an important part of a balanced diet, according to the MyPlate Plan published by the Dietary Guidelines for Americans. All dairy goodness begins with milk production, where the focus is on bringing nutritious, delicious, and safe milk and dairy products to the marketplace.
Enhancing Pasteurized Milk Quality and Preventing Contamination

Pasteurization was introduced in the United States in the 1890s as a groundbreaking method to protect public health by eliminating highly contagious bacterial diseases transmitted through raw milk (unpasteurized milk). Since then, pasteurization has evolved significantly, with a New Jersey milk plant pioneering the first pasteurizer in the U.S. Over a century later, nearly all milk sold in the United States is pasteurized, with limited exceptions like raw milk. (Source: Wikipedia®)
The pasteurization process remains a cornerstone of the U.S. dairy industry, helping to ensure a safe and reliable milk supply. However, maintaining pasteurized milk quality requires addressing several challenges beyond the pasteurization stage.
Factors Affecting Pasteurized Milk Quality
While pasteurization effectively eliminates harmful pathogens, other factors can compromise milk quality, including:
- Milk fat oxidation: Contributing to undesirable flavors.
- Elevated somatic cell counts: Indicating poor milk production conditions.
- Feed-related off-flavors: Resulting from dietary influences on cows.
- Microbial spoilage: The most critical factor impacting pasteurized milk quality.
Understanding Microbial Spoilage Risks
Microbial spoilage in pasteurized milk occurs in two primary ways:
- Heat-resistant psychrotrophic bacteria: These bacteria typically originate from raw milk and can survive the pasteurization process. They pose challenges under certain conditions (to be explored in a future blog).
- Post-pasteurization contamination: This is the most frequent and significant threat to milk quality, often caused by gram-negative bacteria introduced within the processing plant. Ensuring fluid milk safety at this stage is critical to preserving milk and dairy product integrity.
The Importance of Post-Pasteurization Contamination Control
To combat post-pasteurization contamination, employing effective aseptic sampling in dairy plants is essential. This process detects contamination early, helping to prevent spoilage caused by psychrotrophic bacteria, which thrive under refrigeration temperatures and contribute to reduced shelf life and off-flavors.
Bacteriological standards in raw vs pasteurized milk
The chart below shows the standards for Grade “A” milk (Source: “Basic Dairy Bacteriology” published by the Department of Food Science at Cornell University).
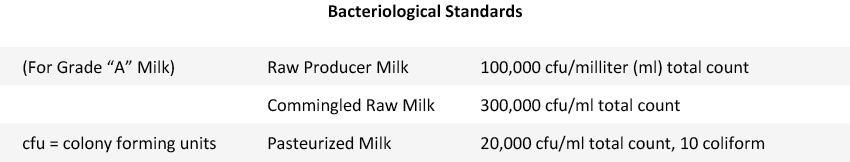
Pasteurization (161°F for 15 sec.) is designed to destroy most potential pathogens in raw milk, substantially reducing harmful bacterial counts for most freshly pasteurized milk to less than 500 cfu/ml, which is far lower than the standard of 20,000 cfu/ml.
Unless proper preventive measures are taken at the dairy processing plants, contamination can occur through post-pasteurization contaminants (PPC). Understanding potential sources and following preventive are essential to the quality and safety of fluid milk.
How Dairy Plants Identify and Minimize Contamination Risks
To help ensure the quality and safety of fluid milk, dairy plants must routinely monitor and test for microbial contamination. This practice is essential for two key reasons:
Regulatory Compliance: Dairy plants are required to adhere to strict regulations, including testing for lab pasteurized counts, coliform counts, and initial standard plate counts. These tests help verify that the production process meets food safety standards.
Quality and Safety: Regular monitoring and testing help ensure consumer trust by delivering milk and dairy products that meet high standards for quality and safety. Consistently safe products reinforce consumer confidence in the dairy industry.
The Role of Sanitation and Process Monitoring in Minimizing Contamination
A robust plant sanitation program combined with regular process monitoring are critical to reducing the risks of microbial spoilage. Common contamination risks in dairy plants include:
- Cracked pasteurizer plates: Allowing raw milk to mix with pasteurized milk.
- Contaminated storage tanks: Creating environments for harmful bacterial growth.
- Airborne contaminants: Introducing microbes during product handling.
- Inadequate personal hygiene: Contributing to the transfer of harmful bacteria during production.
Compressed Air: A Hidden Contamination Risk
Compressed air systems are often overlooked as a source of contamination in dairy plants. Malfunctioning compressed air dryers can introduce microbial contaminants into the production process, posing risks to product safety and quality. Monitoring and maintaining compressed air systems are vital steps in minimizing contamination risks.
Proactive Monitoring and Control
Effective monitoring and control of all potential risks—whether from equipment, air systems, or personnel—are essential for maintaining milk and dairy product quality. Tools like aseptic sampling in dairy plants enable real-time detection of microbial threats, allowing dairy plants to address issues before they compromise product safety.
COOL FACT: One way the dairy industry saves energy involves using the heat of the heated pasteurized milk to warm the next batch of cold raw milk. Cold milk is then used to cool the heated pasteurized milk. By doing this, the industry uses heating and refrigeration energy more efficiently during the milk pasteurization process. (Source: usdairy.com)
Frequently Asked Questions About Maintaining Pasteurized Milk Quality
Q1: What is post-pasteurization contamination, and why is it a concern?
A: Post-pasteurization contamination occurs when microbial contaminants, such as psychrotrophic bacteria, are introduced after pasteurization.
Q2: How can dairy plants prevent microbial contamination?
A: Dairy plants can minimize contamination risks by implementing robust sanitation and monitoring programs that include aseptic sampling and testing to monitor quality at critical control points.
Q3: Why is aseptic sampling important in dairy processing?
A: Aseptic sampling helps ensure contamination-free sample collection, providing accurate data for microbial testing. This helps detect risks early and maintain pasteurized milk quality.
Q4: What are psychrotrophic bacteria, and how do they impact milk quality?
A: Psychrotrophic bacteria are microbes that grow at refrigeration temperatures. They can cause spoilage, off-flavors, and shortened shelf life, even after pasteurization.
Importance of aseptic and representative sampling in process monitoring
QualiTru has a proven method for aseptic and representative sampling used for process monitoring in plants worldwide. The National Conference on Interstate Milk Shipments (NCIMS) and the Food & Drug Administration (FDA) have approved QualiTru sampling products for collection of the milk producer’s “Universal” Dairy Farm Milk Samples under Section 6 of the Grade “A” Pasteurized Milk Ordinance (PMO). (See Regulatory Approvals).
The QualiTru sampling system emphasizes the importance of sampling at critical control points throughout the process. When coupled with standard approved laboratory methodologies, this sampling method can be used to identify the sources of contamination. Aseptic sampling and aseptic testing techniques are both necessary for processors to have confidence in their data.
As we like to say around here, “Your Test Result Is Only as Accurate as Your Sample.”
How does microbial testing determine contamination sources in pasteurized fluid milk?
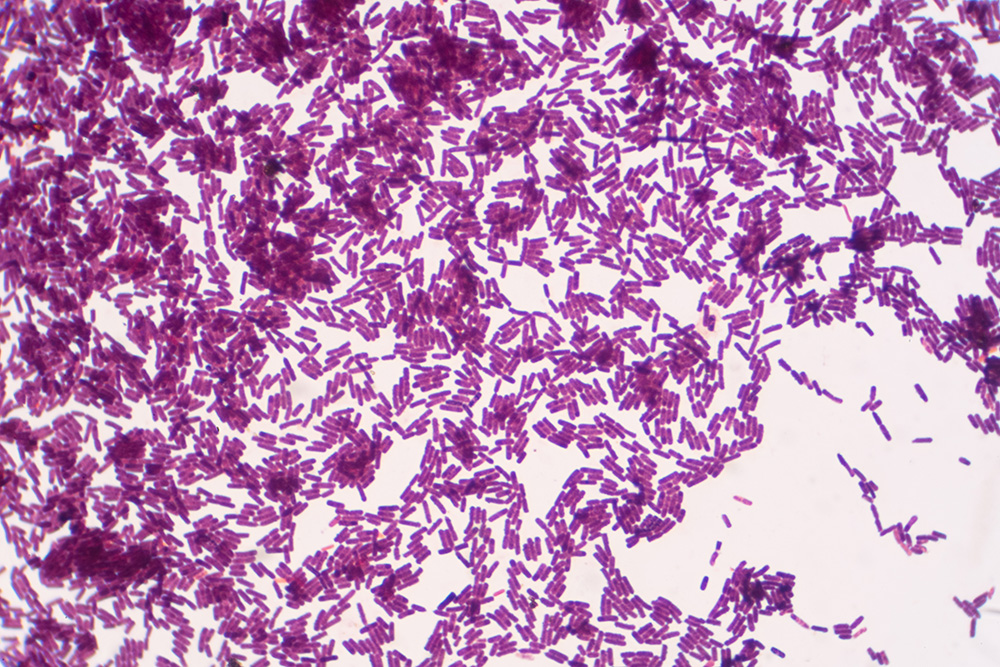
The Mosely Keeping Quality Test is one of several industry-standard tests for the detection of microbial spoilage and other defects. The test requires pasteurized milk to be held for seven to ten days at 45 degrees F., tasted for flavor, and then plated for bacteria. Bacteria counts in the millions per milliliter indicate the need for further analysis.
Gram-staining from the plate counts can identify bacterium types and help further verify the source of spoilage. Post-pasteurization contamination can include both gram-negative and gram-positive bacteria. Gram-positive bacteria could be a potential, though unlikely source, of post-pasteurization contamination.
The presence of any gram-negative bacteria typically indicates contamination in the process. Most psychotropic bacteria are gram-negative and grow at a faster rate at refrigeration temperatures; their presence is most likely due to post-pasteurization contamination. In addition to the spoilage potential of gram-negative bacteria, their presence can also indicate the potential for pathogenic bacteria, which can result in food safety concerns.
Read our case study on Sanitation Verification Using Aseptic Sampling.
Ready to enhance your dairy plant’s quality control processes? Contact QualiTru today to learn how our aseptic sampling systems can help you maintain the highest standards of pasteurized milk quality and fluid milk safety.