Aseptic Sampling for Nutritional Food Supplement Contamination
As with liquid nutraceuticals and functional foods, foodborne illness in dietary and nutritional food supplements presents a serious risk, particularly for elderly individuals, immunocompromised patients, and others who depend on these products for essential nutrition. Distribution of these products to hospitals, long-term care facilities, and home healthcare settings makes ensuring their microbiological safety particularly critical. Implementing robust contamination prevention strategies, including aseptic in-process sampling, helps manufacturers identify microbial hazards early, maintain compliance with food safety regulations, and protect public health from contamination risks.
Understanding Nutritional Food Supplement Contamination Risks
Dietary and nutritional supplements require strict contamination controls due to their formulation complexity, storage requirements, and vulnerability to bacterial contamination. Unlike powdered or tableted formulations, liquid nutritional food supplements often require strict cold-chain management to inhibit bacterial growth. Any lapse in hygiene or processing integrity can introduce harmful pathogens, increasing the risk of contamination. Among the most concerning contaminants are Listeria monocytogenes, Salmonella spp., and Escherichia coli, all of which can originate from:
- Processing equipment contamination, often introduced through transient surface contaminants or biofilms hidden in the process stream.
- Raw materials entering the process stream
- Environmental biofilms that form in drains, floors, walls, and overhead fixtures
- Impure water used in formulation, processing, or cleaning
2025 Listeria Outbreak in Supplemental Shakes Claims 12 Lives
A recent recall of Lyons ReadyCare and Sysco Imperial Frozen Supplemental Shakes in the United States highlights the severity of microbial contamination risks in nutritional supplements. The products were found to be contaminated with Listeria monocytogenes, a pathogen responsible for causing severe illness, particularly in vulnerable populations. Tragically, this contamination has led to 12 deaths across 21 states, underscoring the critical need for strict safety measures in the production of liquid dietary supplements. (Source: Centers for Disease Control, February 24, 2025)
The Role of Aseptic Sampling in Controlling Contamination
Aseptic sampling is a critical component in maintaining the microbial safety of liquid functional foods and dietary and nutritional food supplements. Contamination can enter at multiple stages of production, from raw materials to packaging. Sampling at critical control points in the process allows manufacturers to identify microbial hazards early, take corrective action before products reach consumers, and verify that mitigation steps such as pasteurization and sanitation are effective. Without proper aseptic sampling, contamination may go undetected, resulting in costly recalls, reputational harm, and serious public health risks.
Benefits of Aseptic Sampling with QualiTru:
- Early Detection – Isolates contamination hotspots to safeguard quality and avert potential outbreaks.
- Regulatory and Customer Standards – Validates adherence to food safety standards and customer requirements.
- Process Verification – Confirms that critical contamination control measures, such as pasteurization and sanitation, are functioning as intended.
- Labor Efficiency – Reduces manual sampling workload, allowing personnel to focus on higher-value tasks.
- Safe with Lower Operational Costs – Eliminates unsafe and labor-intensive steam sterilization procedures and reduces cost per test.
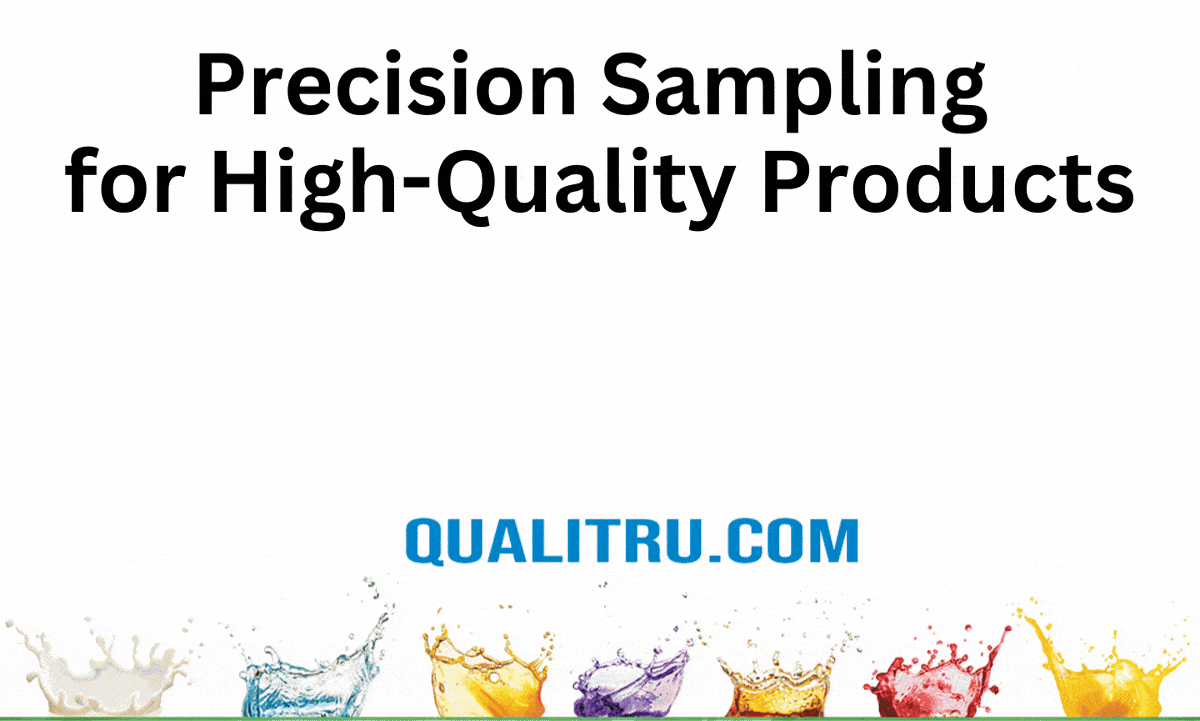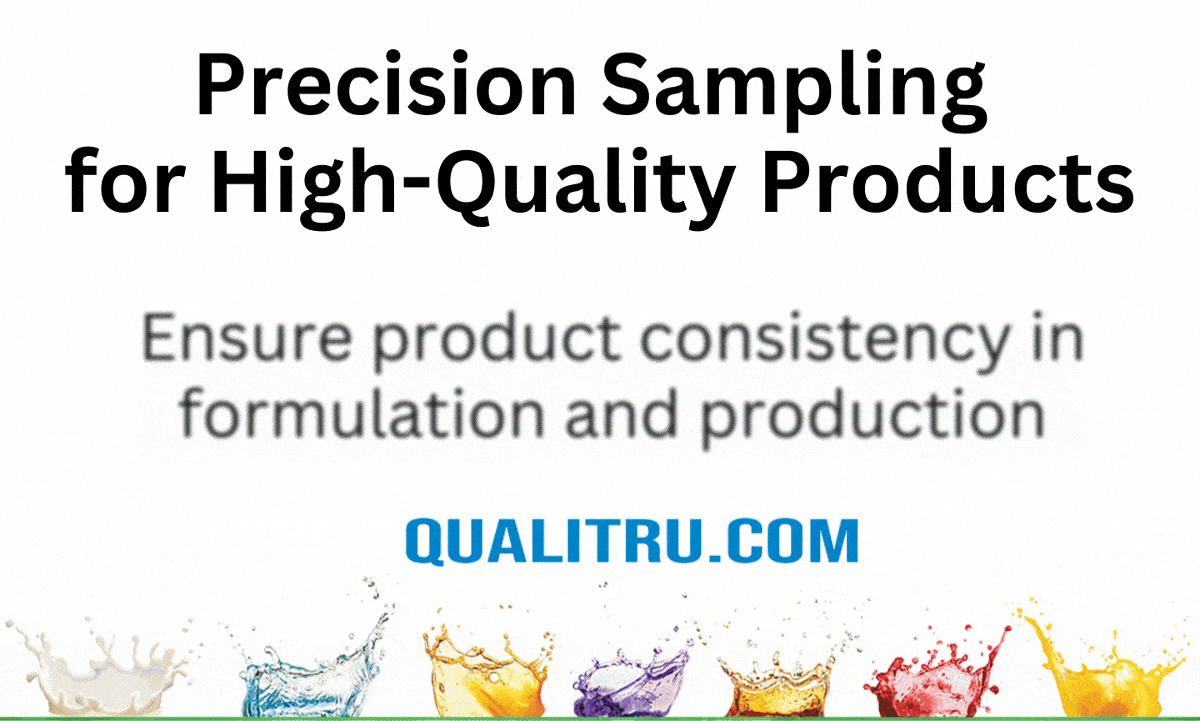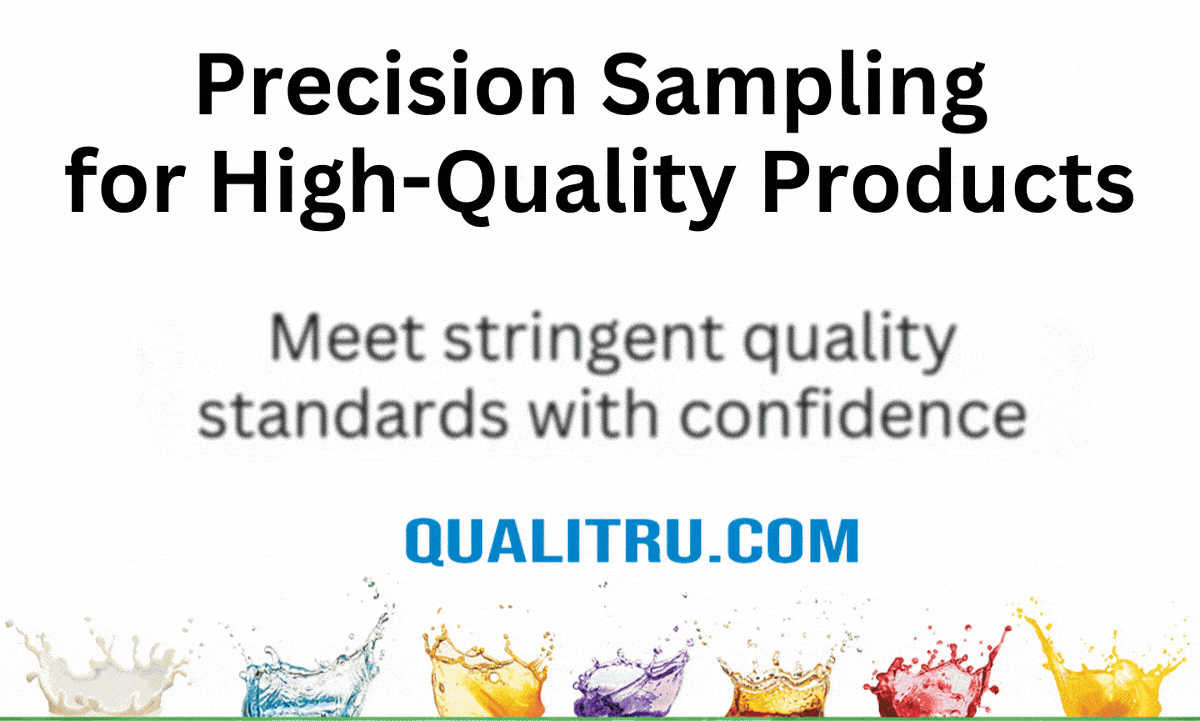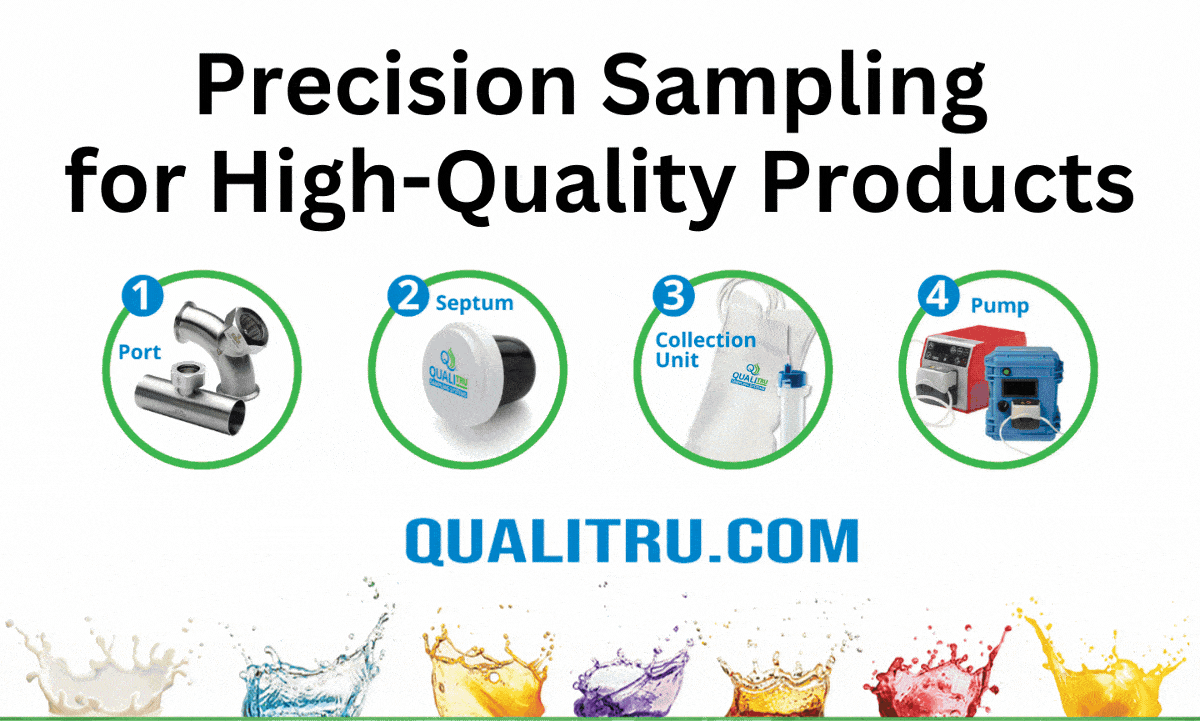
QualiTru’s Aseptic Sampling Solutions
QualiTru offers advanced aseptic sampling systems designed to integrate seamlessly into existing production lines, enhancing microbial monitoring and control.
- Pre-sterilized TruStream™ Septa: Facilitate sterile, multi-use sampling with minimal operational disruption.
- TruDraw® Sterile: A single-use, aseptic sampling solution designed to provide a controlled chain-of-custody and minimize cross-contamination risks.
- Non-invasive, Multi-Point Sampling: Allows for representative sampling across different production stages without compromising product integrity.
Strengthening Food Safety in Nutritional Food Supplements
Maintaining the microbiological safety of liquid functional foods and dietary and nutritional supplements is essential to public health. Implementing comprehensive aseptic sampling strategies helps manufacturers detect contamination early, maintain compliance with regulatory and customer standards, and ensure the safe production of high-quality products. By adopting QualiTru’s aseptic sampling solutions, manufacturers can strengthen their food safety measures and safeguard the well-being of those who depend on these essential nutritional products.
Protect your nutritional food supplements from contamination. Contact us today to learn how our QualiTru can help safeguard your dietary and nutritional supplement monitoring processes.
QualiTru’s aseptic sampling solutions offer a simple, fast, and versatile way to monitor for microbial contamination. Engineered for seamless integration, these pre-sterilized, ready-to-use systems enhance process control, regulatory compliance, and product integrity, ensuring the highest standards of safety in liquid dietary supplements.
- Inline Sampling
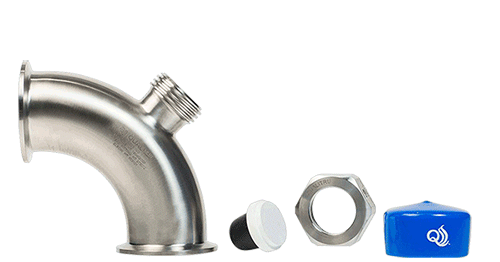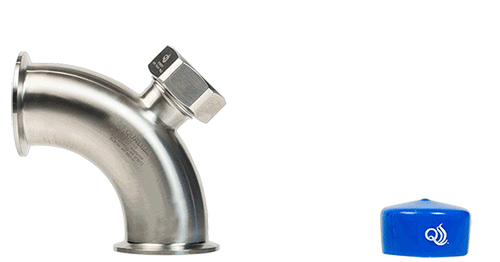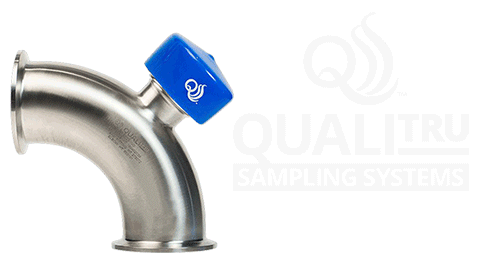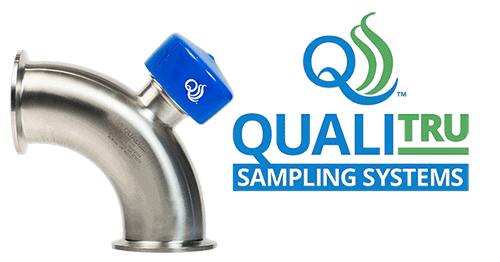
- TruStream7 Tri-Clamp Tee 2” (Part #215147) or TruStream 7 Tri-Clamp Elbow 2″ (Part #213029)
- TruStream7 Septa (Part # 110011)
- TruStream 250ml/18g (Part #111450) or TruMotion 2L/18g w/2.0mm Tubing (Part #111770) with a Watson-Marlow Pump (Part #500000) for a representative sample or the TruDraw® Sterile Single Sampler (Part #112021) for a small, aseptic sample
- Holding Tank Sampling
- TruStream7 Recessed 4”x 2” (Part #212123)
- TruStream7 Septa (Part #110011)
- TruDraw® Sterile Single Sampler (Part #112021)
Have questions about sampling for nutritional food supplement contaminants?
Need a quote? We’ll respond to
your request quickly.
Looking for one of our global distribution partners near you?