Effective Micro Program: Understanding the Microbiological Tests (Part 4)
This is a guest post written by Neil Bogart, owner and president of Bogart Food Safety & Sanitation Associates, Inc. He is a highly respected adviser in the food and beverage industry, providing troubleshooting, technical support, cleaning, and sanitizing solutions to the dairy, food, beverage, and bottled water markets. See Author Bio at the end of the post.
Part 4Topic: Effective Micro Program: Understanding the Microbiological Tests
Throughout this series on dairy microbiological testing, we have discussed the types of micro tests that can be used to enumerate the classifications of bacteria we are concerned with in fluid milk processing. The first two articles discussed Gram-negative bacteria in raw milk and the effects of Gram-positive heat resistant thermoduric/psychrotrophic spore formers (HRSF) on fluid milk shelf life. In the third article, Micro Testing for Post-Pasteurization Contamination, we looked at the common micro-testing options for post-pasteurization contamination, the pros and cons of these tests, and the reasons for utilizing the tests. Answering the question, “Why are you using this specific test and what is it telling you?” is a critical first step in understanding your micro issue. In this fourth and final installment, we combine what we learned about these tests to develop an effective micro program to support your desired shelf life and processing methods.
Building an Effective Micro Program
Just as with Corrective and Preventive Action (CAPA), we need to utilize a methodical process to determine the root cause of an issue; this is the same with our micro-testing programs. Therefore, when building a micro program, we want to design it with the least number of tests that yield the biggest payout of information in the quickest amount of time. The lab’s customers, production, sanitation, and maintenance need information on how all their programs support the finished product’s quality and safety. If there is a failure in one of their programs, like Preventive Maintenance (PM), that impacts completion on time, there could be a direct effect on the quality and safety of the product.
Below I have outlined a recommended program for fluid milk facilities that are trying to achieve an 18+ days shelf life expiration code on conventionally processed milk. For the purpose of this blog series, I am focusing on micro tests only. If you would like to further develop a comprehensive plan specific to your process, do not hesitate to contact me.
Raw Milk Receiving
As we learned in the first article of the series, the first line of defense for microbiological testing is at receipt of the raw milk. There are tests used to determine the milk microbiological quality prior to receipt and historical tests used to further determine the milk quality after receipt. The historical tests are used to monitor the farms’ micro and work with them on improving their Good Milking Practices. These tests together constitute the micro program. Other tests that need to be run, that are not part of the micro program and have not been discussed as part of this series, include antibiotics, freezing point, and chemistry at receipt of the milk prior to accepting it.
Pre-Receipt Tests:
- Titratable Acidity (TA) – Every Load (action level >0.17% Acidity – – Goal 0.15 – 0.16% Acidity) (R. Hooi, 2004)
- Direct Microscopic Count (DMC) – Every Load (action level >100,000/mL – – Goal <50,000/mL) (Laird, 2004)
Historical Tests:
- Preliminary Incubation (PI) 1:1000– Every Load (action level >50,000 cfu/mL – – Goal <20,000 cfu/mL)
- Gram-positive HRSF Test – Every Load (action level >100 cfu/mL); or,
- HR-3 Test – Every Load (action level – White)
- Rope Milk Test – Every Load (action level – Positive – – Goal – Negative)
In-Process
After ensuring we have reduced the risk of accepting poor-quality raw milk at receiving, we must make sure the quality is maintained throughout processing. You can do a lot of work to receive good quality raw milk, but if it is not maintained, the quality of your finished product can be significantly and quickly reduced. The following tests will allow you to understand what is happening to the raw and pasteurized milk throughout the process. You can do this by focusing on the High Temperature Short Time (HTST).
HTST Balance Tank & Discharge: Weekly
Balance Tank Sample:
- PI Test 1:1000 (action level >50,000 cfu/mL)
- Gram-positive HRSF Test – Psychrotrophic count; or,
- HR-3 Test (Qualitative/Quantitative Test)
Discharge Sample:
- Gram-positive HRSF Test – Psychrotrophic count (action level, no significant difference between balance tank and discharge); or,
- HR-3 Test (action level, no significant difference between balance tank and discharge)
Finished Product
Now that we understand what the potential quality of fluid milk is coming out of the HTST, we must ensure the quality is maintained from the discharge of the HTST into the bottle. There is a lot of equipment along this path, including two valve clusters, pumps, silos, and miles of piping. The following tests will allow you to understand what is happening to the pasteurized milk on the path from the discharge of the HTST into the bottle.
- Shelf-life Sensory Analysis @ 7.2°C (45°F)
- Fresh coli 1:1 (action level >1 cfu/mL)
- Stress SPC 1:1 (action level >100 cfu/mL)
- Stress coli 1:1 (action level >1 cfu/mL)
- Stress PPC 1:1 (action level – monitoring)
Implement the accelerated testing protocol if the Fresh coli, Stress SPC, or Stress coli Test starts entering action level counts.
Accelerated Testing Protocol
The accelerated testing protocol is used to break down the plant into manageable sections so we can start to understand at which point in the process micro-contamination is introduced. One method for monitoring this process is with QualiTru TruStream™ aseptic sampling ports installed throughout the post-pasteurized side of the process, including at the discharge of the HTST, in and out of silos, and just before the fillers.
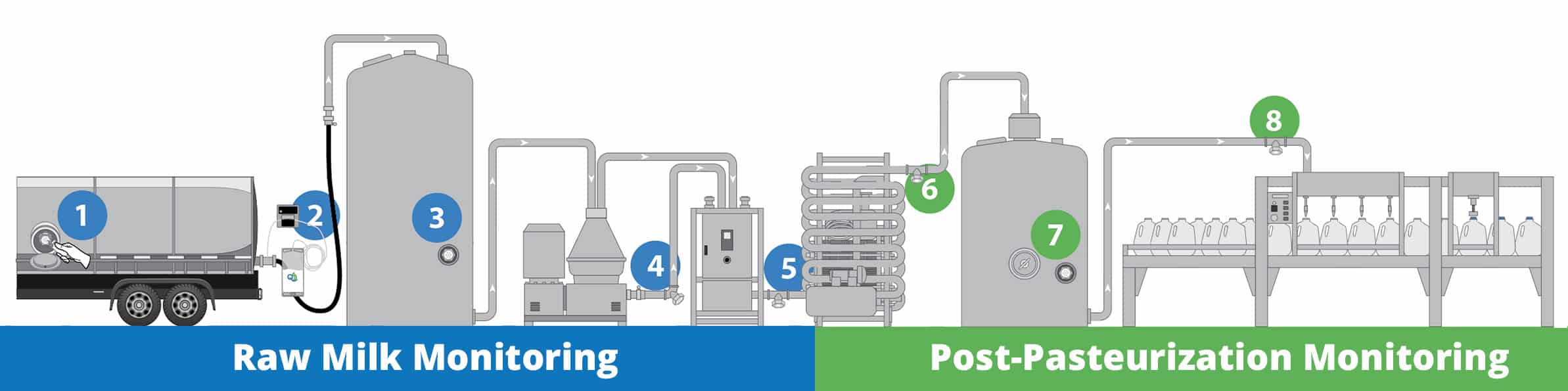
Start by line sampling at the in and out of silos and just before the fillers utilizing sterile QualiTru TruStream™ 250mL/18g collection bags. Utilize the following micro tests to help identify at which point in the process the micro contamination starts showing up.
- Stress SPC 1:1 (action level >100 cfu/mL)
- Stress coli 1:1 (action level >1 cfu/mL)
- Stress PPC 1:1 (action level – monitoring)
Ensure Accuracy and Precision for Product Safety and Quality.
By implementing a methodical process in the microbiological program, quick results can be delivered with the understanding of the potential source of the contamination to the lab’s customers, production, sanitation, and maintenance. This method is far superior to taking a generic approach, “We have high micro counts out there.” “Out there” means miles of pipes and many pieces of equipment that could be the cause of the contamination. With today’s limited resources, we must deliver more accurate and precise results by limiting the potential source to an isolated area and acting responsibly and in an expedited manner. An effective micro program is the first critical step in obtaining desired shelf life, especially when trying to achieve a shelf life code greater than 14 days. The micro program must be accompanied by Preventive Maintenance (PMs), Good Manufacturing Programs (GMPs), Master Sanitation Schedule (MSS), and pasteurization temperatures. Those can all be discussed in a future series.
Additional resources related to dairy micro testing for post-pasteurization contamination:
Articles
Looking Where it Matters
Sampling: Back to Basics
In-Line Fluid Sampling Without Contamination
Previous blog posts in this series:
Raw Milk Dairy Micro Testing (Part 1)
The Effects of Gram-positive HRSF Bacteria on Fluid Milk Shelf Life (Part 2)
Micro Testing for Post-pasteurization Contamination (Part 3)
Training
Why Process Monitoring & Aseptic Sampling Information Sheet
Training Videos
Printable Application Site Schematics

Finding solutions for contamination issues can be challenging, frustrating, time-consuming, and sometimes costly! If you have a contamination issue you are having trouble solving or would like to discuss a proactive process monitoring plan, contact us at (651) 501-2337 or complete our convenient “Ask Our Experts” form to have one of our experts contact you.
Reference: Laird, J. E. (2004). Direct Microscopic Methods for Bacteria or Somatic Cells. In a. H. M. Wehr, Standard Methods for the Examination of Dairy Products, 17th Edition (pp. 269 - 280). Washington, DC: American Public Health Association. R. Hooi, D. M. (2004). Chemical and Physical Methods. In aH. M. Wehr, Standard Methods for the Examination of Dairy Products, 17th Edition (pp. 363 - 366). Washington, DC: American Public Health Association.




