Membrane Filtration Sampling
Membrane filtration is a widely used process in the dairy industry that separates specific components of milk and whey and then either concentrates or removes/reduces them. The technology used in cheese-making, whey protein concentration, fractionation of protein, and numerous other dairy processes requires special sampling considerations for both regulatory purposes and component analysis. It is critically important to create an integrated membrane filtration sampling program that you can trust to monitor process health and product quality. To do so, it is important to understand sampling issues unique to membrane hygiene.
Challenges of Microbial Monitoring Within a Filtration System
A filtration system is more complex than a pipeline for microbial monitoring. It provides hiding places for bacteria to nest and exponentially propagate and/or produce exopolysaccharides, a mucoid polysaccharide matrix produced by bacterial colonies, such as those in biofilms, that protects against heat, antibiotics, and other chemicals in the presence of nutrients. This leads to the following side effects and impacts.
First, there is a reasonable chance that bacteria can and will collect in the concentrate side of your system, even with all the cleaning prevention done upstream. The most common side effect of bacteria buildup is blockage or an increase in pressure drop in the first stage. This can have a snowballing effect with the production rate dropping quickly. Usually, the run will take longer, which means the clean-in-place (CIP) window gets tighter and pinched towards the end of the day and may barely pass standards. The bacteria buildup can have a compounding effect as each day means wear filings are pounded in. It makes each successive run harder.
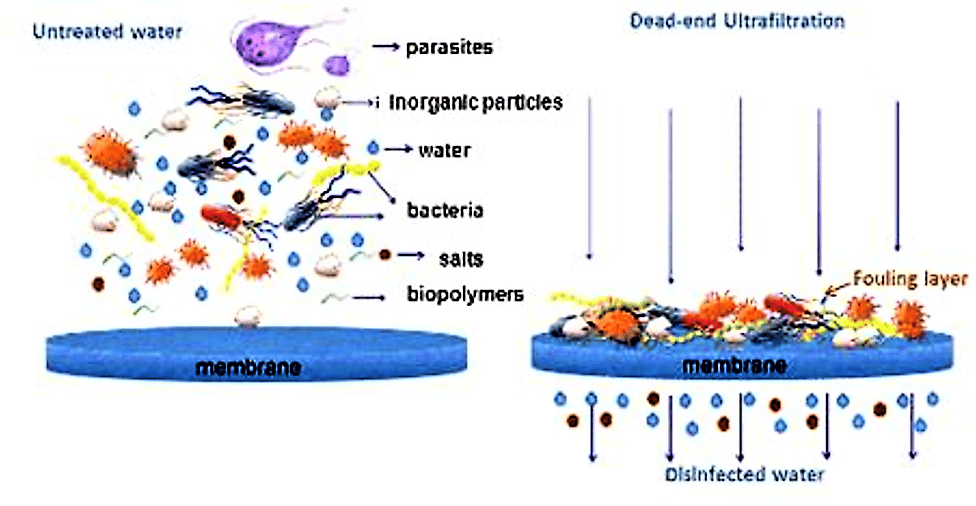 Scheme of membrane filtration: during ultrafiltration all particles and pathogenic microorganisms are retained on a porous membrane while water and dissolved salts pass through. (Source: SSWM University)
Scheme of membrane filtration: during ultrafiltration all particles and pathogenic microorganisms are retained on a porous membrane while water and dissolved salts pass through. (Source: SSWM University)
Second, operating temperature is specific to your plant, driven by regulatory, flux rate demands, or the sensitivity of your streams. For optimum filtration performance, you should be inside a cautionary range of around 15 to 50 degrees Celsius for bacterial growth management. It’s critical to have a sampling system that accounts for this. I’d encourage anyone to examine what your membrane supplier has in terms of autopsy options by pulling the elements, and returning them in original packaging, so that you can learn more extensively about critical aspects of your elements and what may be hidden concerns.
Third, unwanted fermentations may happen, even if the flux appears strong. For example, if organic acid is generated, pH could drop substantially. That would affect functionality or flavor, and possibly even the behavior of your products or process streams downstream.
Aseptic Sampling Systems in Process Monitoring Help Solve Membrane Filtration Sampling Challenges
There is a definite risk of contaminating or affecting the downstream process by using simpler, older sampling methods such as twist-handle sample spigots or the spring-push sample spigots. Even sample spigots with an actuator on them have drawbacks.
When a company is serious about NOT having any cross-contamination from inline sampling, it cannot take the chance of residue remaining in the sample valve body from processing that does not get cleaned and sanitized during CIP/steam-in-place (SIP) steps. There is also the potential to siphon back unfiltered air with yeast/mold, residue, bacteria, etc. from a sample valve if the pressure changes in the process stream. Some valves can be manually opened for sampling and an airline can be set up to automatically open during cleaning, but that means cleaning solution that shoots out the spigot must be captured in some sort of basin to be safe. No one likes hazardous chemicals being sprayed onto the floor during a night shift when most facilities clean their process line. These systems are also difficult to clean thoroughly and may not be routinely inspected.
Aseptic sampling systems are ideal for multi-use inline sampling throughout the membrane filtration process because they do not ‘disturb’ downstream sampling points or the process in general. The risk of contaminating the sample is effectively mitigated due to the design of single-use channels in the sanitary septum.
Aseptic sampling allows for the identification of low levels of bacteria that will concentrate to a more problematic level in the future. Inline sampling allows for a reaction, a response to act early to fix a problem before it prevents the system from operating and promotes critical awareness of bacterial levels that build over time. Plants can sample and correlate operating temperature to the effects of fouling and pressure drop, as well as bacterial count. They can then assign a trigger for extra cleaning cycles if they see a bacterial level during a routine test that correlates with an imminent problem.
An Example of an Aseptic Sampling System for Microbial and Compositional Analysis
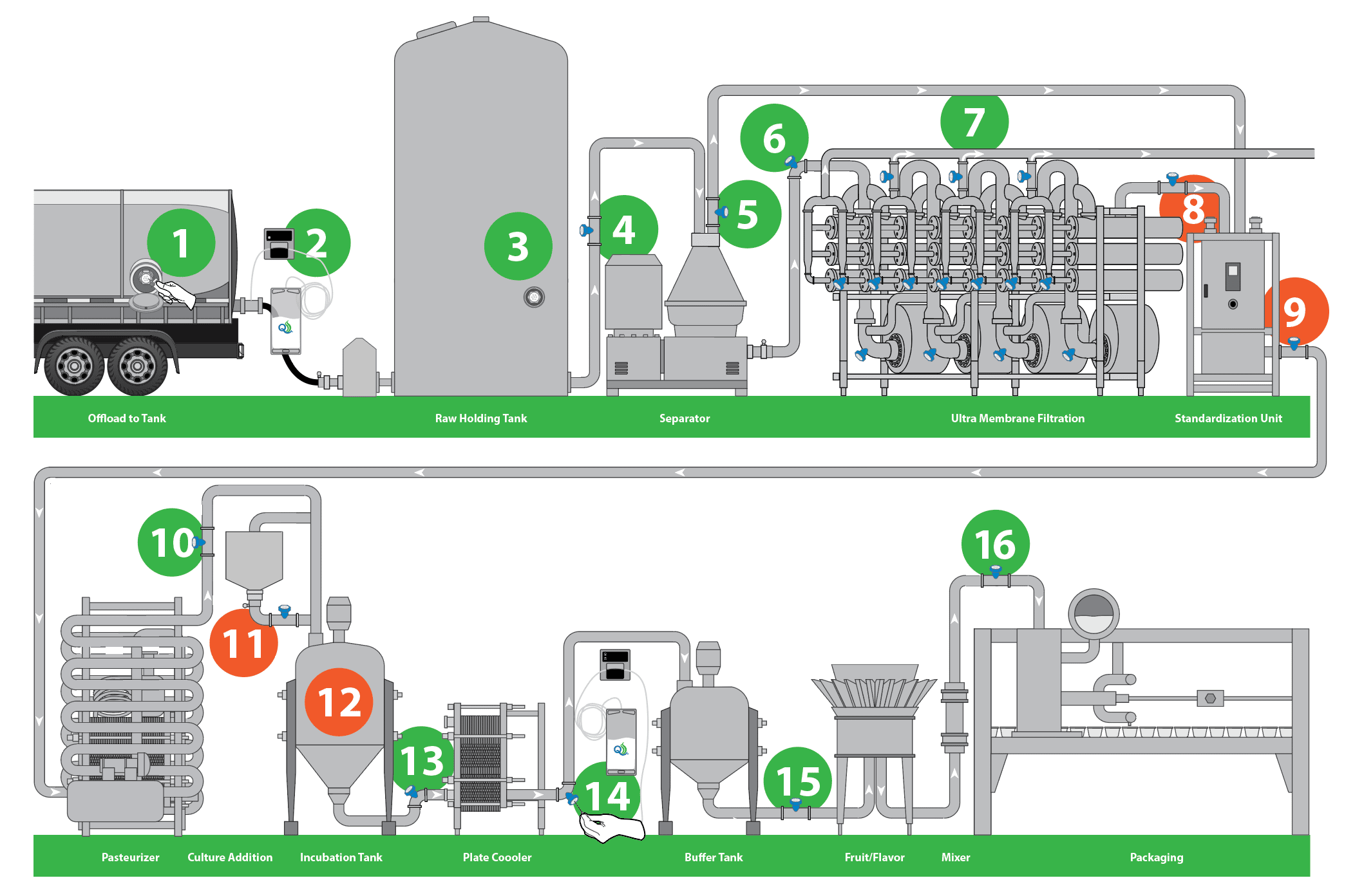
The following diagram shows critical aseptic sampling sites used for both microbial and compositional analysis within a Greek yogurt manufacturing process. Some sampling sites would only be used for composition and others would be used to capture microbial data. You can also get multiple points of data from each sample, as well as use the sampling method system for the purpose of inoculation.
The diagram starts with unloading a raw tanker into a silo storage, where you usually need both compositional and microbiological testing. You can separate and concentrate and be able to take a skim or cream stream, split it up and go into ultrafiltration, where you need to know your sample composition, while staying aware of bacteriology. You would have the standardization box that we see around #8 and #9, feeding into the pasteurizer or hold tubes, where it’s important to have bacterial analysis.
Downstream from that sample at #11 and #12, is the site of culture additions and the inoculation tank. Because the system is closed to environmental elements and each channel on the sterile sampling septum is only used once, it is ideal for injecting cultures while mitigating any risks of cross-contamination or introduction of bacteriophage.
Further downstream of the fermentation site, it is critical to monitor the cleaning processes and make sure all the plates are clean. For the Greek yogurt manufacturer, the system can be used to add flavors or other additions at this site. Sampling at these sites will also be used for compositional and microbial testing to ascertain that targets are being met just prior to filling.
Taking Membrane Filtration Sampling to the Next Level
Membrane technology presents unique sampling challenges for regulatory and component analysis. An aseptic sampling system that accommodates specific plant needs allows for multiple-use sampling along the manufacturing process while mitigating the possibility of introducing contamination. Our “From the Field: Practical Tools for Membrane Process Monitoring and Filtration Verification” below provides a deeper dive into membrane filtration sampling, including:
- System diagnosis and operation
- How to capture meaningful data
- Membrane rejection basis (batch and multi-loop)
- Approaching sampling to create calibrations for compositional testing
- And more!
Additional Resources:
- Free on-demand webinars:
Webinars and Training Video - Related blog posts:
Clean in Place (CIP) Troubleshooting blog
How Bacterial Generation Times Impact Fluid Milk Quality and Shelf-life - Training
Site application schematics
Standard Operating Procedures (SOPs)
Make better business decisions with a true aseptic and representative sampling system from QualiTru.
Have questions about aseptic and representative sampling? Ask Our Experts