Preventing Ice Cream Contamination with Aseptic Sampling
Ice cream, a beloved treat worldwide, can be a source of serious foodborne illnesses if contaminated with harmful bacteria. Contaminants such as Salmonella, Listeria, E. coli, and Gram-positive bacteria can find their way into ice cream if rigorous safety measures are not in place. To prevent contaminated ice cream during production, it is crucial to regularly verify cleaning and sanitation processes and employ comprehensive inline process monitoring. These practices are essential for maintaining the highest levels of hygiene and food safety.
Common Bacteria in Ice Cream: A Chilling Hidden Threat
Ice cream, with its creamy texture and delightful flavors, can unfortunately become a breeding ground for various harmful bacteria if production processes are not meticulously monitored and controlled. The presence of bacteria such as Salmonella, Listeria, E. coli, and even Gram-positive bacteria in ice cream can lead to spoilage or severe foodborne illnesses, posing significant health risks to consumers. These bacterial contaminants can enter the product at various stages of production, from raw material handling to processing and packaging. Hence, it is crucial to implement rigorous food safety protocols, including inline aseptic sampling, to detect and mitigate these risks effectively.
Salmonella: The Silent Invader
Salmonella is a leading cause of foodborne illness linked to contaminated ice cream. This bacterium thrives in environments with poor hygiene practices. Contaminated ingredients or surfaces can introduce Salmonella into the ice cream production process, where it can proliferate if not promptly addressed. Inline aseptic sampling is essential in detecting Salmonella early, enabling manufacturers to take immediate corrective actions. By continuously monitoring for this bacterium, producers can prevent large-scale microbial contamination and protect consumers from potential outbreaks.
Setting a Benchmark: Schwan’s Ice Cream Recall of 1994
In October 1994, Schwan’s Sales Enterprises recalled their ice cream products after a Salmonella outbreak sickened thousands. The contamination was traced to a shipment of raw eggs carried in a tanker that wasn’t properly cleaned before transporting the ice cream pre-mix. In response, Schwan’s ceased all ice cream production and issued a national recall, setting a precedent for handling foodborne illness outbreaks responsibly. (Source: The Seattle Times)
Listeria: A Deadly Contaminant
Listeria monocytogenes is particularly concerning in the context of ice cream production due to its ability to survive and grow at low temperatures. This characteristic allows it to persist in frozen products, leading to listeriosis, a serious infection that can be fatal, especially for vulnerable populations such as pregnant women, newborns, the elderly, and immunocompromised individuals. Regular environmental and product sampling are crucial in identifying and controlling Listeria contamination within ice cream facilities. By implementing stringent monitoring protocols, the incidence of Listeria can be significantly reduced, ensuring safer products for consumers.
A Cautionary Tale: Blue Bell’s 2015 Ice Cream Recall
In April 2015, Blue Bell Creameries faced a massive recall of all its products due to a Listeria contamination that resulted in multiple illnesses and deaths. The contamination was traced to several products, including ice cream, frozen yogurt, sherbet, and frozen snacks, produced in their Texas and Oklahoma facilities. The Centers for Disease Control and Prevention (CDC) confirmed that Listeria monocytogenes had been found in various Blue Bell products, leading to serious health risks for consumers. Blue Bell’s swift recall and subsequent implementation of stringent safety measures were crucial steps in addressing the outbreak and restoring public trust (Source: CNN)
E. coli and Coliform Bacteria: Indicators of Hygiene
The detection of E. coli and other coliform bacteria in ice cream is an indication of contamination and inadequate sanitation practices. These bacteria typically enter the production environment through contaminated water, raw materials, or poor hygiene among food handlers. Inline aseptic sampling systems play a vital role in the early detection of these contaminants. By identifying the presence of E. coli and coliforms promptly, producers can prevent the distribution of contaminated batches to the market.
Gram-Positive Bacteria: A Persistent Problem
Gram-positive bacteria, such as Bacillus cereus and Staphylococcus aureus, are not typically found in ice cream. However, they pose a unique challenge in ice cream production. These bacteria can produce toxins that remain active even after pasteurization, posing a continuous threat. Bacillus cereus can cause gastrointestinal distress, while Staphylococcus aureus can lead to severe food poisoning. Continuous monitoring through aseptic sampling helps in detecting these bacteria before they reach harmful levels. By identifying contamination early, producers can take steps to eliminate these bacteria and their toxins, ensuring the safety and quality of their ice cream products.
From Crisis to Solution: Tackling Ice Cream Contamination with Precision
David Blomquist, President of DFB Consulting LLC, recently assisted a large dairy plant experiencing a contamination issue of ice cream mix products at the bulk filler. Numerous efforts to solve the problem had failed, resulting in persistent complaints from food service customers. Through inline sampling with QualiTru’s sampling system and testing, Blomquist was able to accurately isolate and identify the spoilage organisms as Gram-positive bacteria that had survived the pasteurization process. Identifying the issue required large aseptic samples to be collected immediately after the pasteurizer to trace the source of the high bacterial counts. Once the source was identified, the plant implemented special cleaning procedures, resolving the ice cream contamination problem and preventing reoccurrence.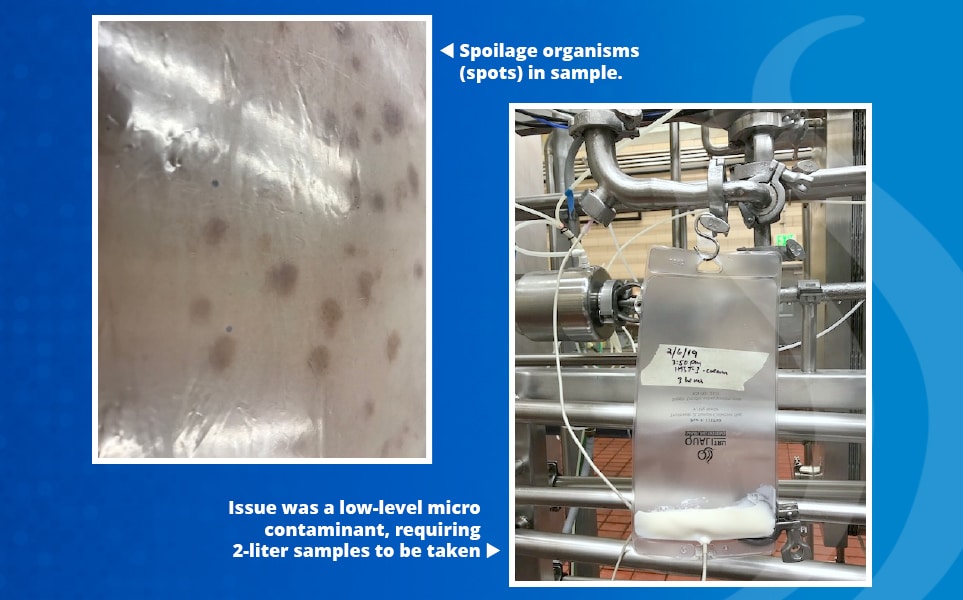
Have questions about aseptic sampling for ice cream contamination?
Need a quote? We’ll respond to
your request quickly.
Looking for one of our global distribution partners near you?
Best Practices to Prevent Bacteria in Ice Cream
Producing safe, high-quality ice cream requires more than just good ingredients—it demands a proactive approach to contamination control. Below are key strategies every ice cream producer should follow to safeguard product integrity and consumer health.
Implement a HACCP Plan for Quality Control
A Hazard Analysis and Critical Control Points (HACCP) plan is vital in ice cream production to identify and control potential hazards. The following key steps can help ice cream producers effectively manage risks and ensure their products are safe for consumption:
- Conducting a Hazard Analysis: Identify potential biological, chemical, and physical hazards that could affect ice cream quality and safety.
- Determining Critical Control Points (CCPs): Points in the production process where controls can be applied to prevent or eliminate hazards, such as pasteurization and mixing.
- Establishing Critical Limits: Set maximum or minimum values for biological, chemical, or physical parameters that must be controlled to ensure safety, like temperature limits for pasteurization.
- Monitoring CCPs: Regularly check and record data to ensure critical limits are being met, ensuring consistent safety and quality.
- Implementing Corrective Actions: Define steps to be taken when monitoring indicates that a CCP is not under control, such as adjusting processing temperatures or handling procedures.
- Verifying Procedures: Validate that the HACCP system is working effectively through testing and review of records.
- Record-Keeping: Maintain documentation and records to demonstrate HACCP compliance and facilitate continuous improvement in food safety practices.
Cleaning and Sanitation Verification: The Backbone of Safety
Verification is the process of ensuring that cleaning and sanitation efforts have been effective. This involves routine testing and inspection to confirm that equipment and surfaces are free from harmful bacteria. Verification methods may include:
- Visual Inspections: Regular checks to ensure that equipment and surfaces appear clean and free from residues.
- Swab Tests: Collecting samples from cleaned surfaces and equipment to test for the presence of bacteria, using methods such as ATP bioluminescence testing or microbial culture techniques.
- Microbial Testing: Regular sampling and testing of the production environment and ice cream products for specific pathogens like Salmonella, Listeria, and E. coli.
Integrate Continuous Monitoring: A Pillar of Quality Assurance
The integration of inline sampling systems into the production process allows for real-time monitoring of critical parameters that affect the quality and safety of ice cream. This continuous oversight is crucial for several reasons:
- Real-Time Data Collection: Inline sampling systems provide instantaneous feedback on the presence of contaminants, ensuring that any deviations from the norm are detected immediately. This real-time data collection allows for prompt corrective actions, minimizing the risk of widespread microbial contamination.
- Consistent Product Quality: By continuously monitoring the production process, inline sampling helps maintain consistent product quality. Variations in ingredient quality, equipment performance, or environmental conditions can lead to inconsistencies in the final product. Inline sampling systems detect these variations early, allowing producers to make necessary adjustments and maintain a uniform standard of quality.
- Enhanced Safety Protocols: Inline sampling ensures that safety protocols are adhered to at all times. The system can identify potential hazards before they escalate, ensuring that only products meeting the highest safety standards reach the consumer. This proactive approach significantly reduces the likelihood of recalls and enhances consumer trust in the brand.
Prevent Contaminated Ice Cream Issues and Ensure Brand Loyalty
By continuously monitoring the production process, inline sampling helps maintain consistent product quality and safety, ensuring that only the best products reach consumers. Contact QualiTru Sampling Systems today to learn more about inline aseptic sampling in dairy plants for quality ice cream production.
Complete this form to have one of our experts contact you to discuss implementing aseptic inline sampling as part of your process monitoring plan! Or call us at (651) 501-2337 or email [email protected] to learn more and/or to discuss your needs.
By facilitating accurate, timely, and reliable sampling, QualiTru systems enable proactive interventions, helping to maintain the highest standards of dairy product safety and quality.
- Inline Sampling
- TruStream7 Tri-Clamp Tee 2” (Part #215147) or TruStream 7 Tri-Clamp Elbow 2″ (Part #213029)
- TruStream7 Septa (Part # 110011)
- TruStream 250ml/18g (Part #111450) or TruMotion 2L/18g w/2.0mm Tubing (Part #111770) with a Watson-Marlow Pump (Part #500000) for a representative sample or the TruDraw® Sterile Single Sampler (Part #112021) for a small, aseptic sample
- Holding Tank Sampling
- TruStream7 Recessed 4”x 2” (Part #212123)
- TruStream7 Septa (Part #110011)
- TruDraw® Sterile Single Sampler (Part #112021)



