Hygienic Sampling Practices Are Vital to Protecting Dairy Product Quality and Safety
Milk collected from the udders of healthy cows is almost sterile but microbial contaminants are introduced during milking, storage, processing, and distribution. Cleaning and sanitation failures allow contaminating organisms to accumulate in dairy processing equipment, potentially leading to the development of biofilms that can become a significant and persistent source of contamination. On the farm and in the dairy processing environment, hygienic sampling practices are vital for identifying sources of contamination to protect dairy product quality and safety.
Biofilm Formation
Biofilms are immobile (or sessile) aggregations of bacterial cells that form on equipment or environmental surfaces. Bacteria making up biofilms are interconnected by extracellular polymeric substances (EPS) that accelerate growth and protect the cells from heat and chemical sanitizers. It is estimated that 40%-80% of all bacterial species on earth are capable of forming biofilms, including all the bacterial species of interest in dairy quality and safety.
Biofilms form in a predictable but complex fashion, beginning when free-floating (or planktonic) cells weakly adhere to a surface and begin to excrete EPS. This is the first in a series of five main phases of biofilm formation that include: (i) the reversible attachment phase; (ii) the irreversible attachment phase, where interaction between surfaces and lipopolysaccharides found on hair-like bacterial appendages (pili or fimbriae) form tight ionic attachments; (iii) production of EPS by the cells forming the biofilm; (iv) biofilm maturation during which cells grow, cell density increases, and cells synthesize and release signaling molecules allowing them to sense and communicate with each other; and (v) dispersal or detachment phase, where the cells depart in large numbers to become planktonic cells again.
The Five Main Phases of Biofilm Development
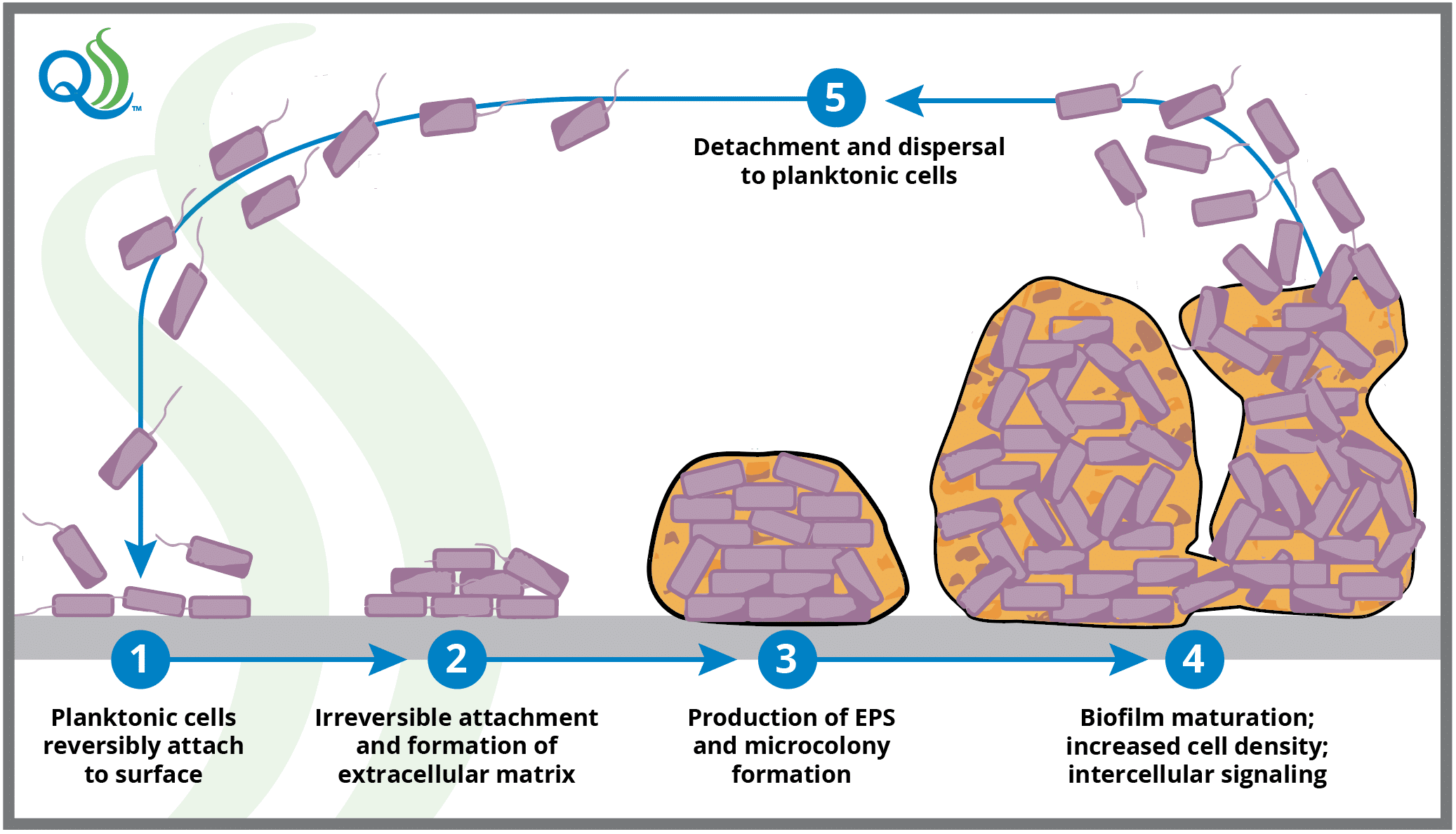
Maturation of biofilms may take 72 to 144 hours or longer depending on bacterial species and growth conditions. Although most bacterial detachment from the biofilm occurs following maturation, cells may detach at any time during biofilm development, making biofilms a potential and persistent contamination risk immediately upon initiation.
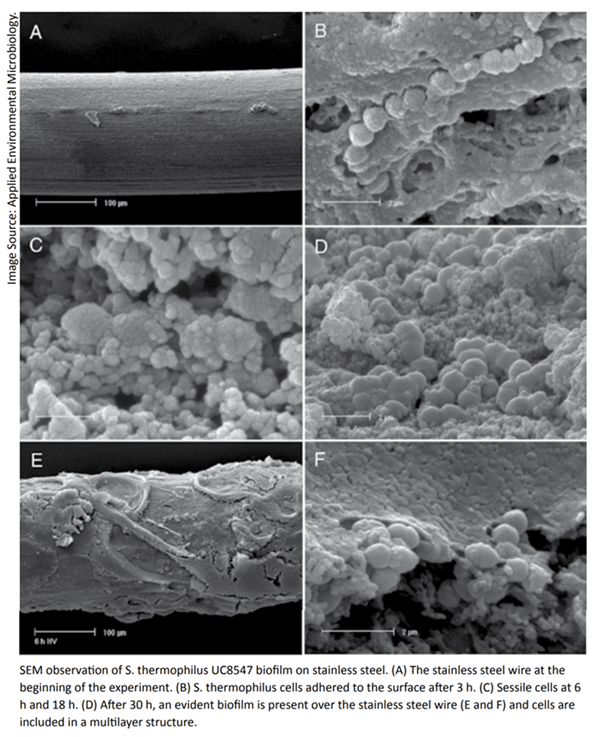 Importance of Biofilms in the Dairy Industry
Importance of Biofilms in the Dairy Industry
Biofilms can form on various materials and surfaces in dairy farms or processing plants. Teflon, rubber, stainless steel, glass, polypropylene, ceramic or other tiles, and most other materials found in dairy production or processing environments will support biofilm formation. As such, biofilms may become established on environmental surfaces, such as walls, floors, and drains; in milking parlors or equipment; inline milk filters; stainless steel equipment, such as milk storage tanks, pasteurizers, and piping; gaskets; valves; tools; milk handling devices; and more.
The widespread nature of biofilm-forming microbes in the dairy industry represents an important source of bacterial contamination affecting raw and pasteurized liquid and dry dairy products. This persistent contamination may result in foodborne disease, food spoilage, damage to brand reputation, and economic loss.
Hygienic Sampling Practices are Essential for Detecting Biofilm Contamination
Detecting biofilms in a milk production or processing plant environment is difficult. Direct observation methods, such as UV light, are limited by the need for open and easily observable surfaces. These direct methods are generally not suitable for finding biofilms in closed equipment environments, such as milk lines, heat exchangers, valves, most milk storage tanks, and other such equipment.
The only sure way to find biofilm contaminants in a complex milk production or processing environment is to isolate contamination hotspots, which are locations within a process where biofilms may form. In-process hotspot isolation methods should be used to monitor process contamination hazards. For example, microbiological testing of aseptic samples collected upstream and downstream of equipment identified as a potential contamination hazard will isolate bacterial contamination originating from the equipment. Equipment that may constitute a contamination hazard may include heat exchangers, inline filters, milk storage or blending tanks, valves and valve clusters, filling machines, evaporators, and others.
Microbiological tests to identify these hotspots may be as generic as total plate or coliform counts or as specific as tests for environmental or contagious pathogens, psychrotolerant spore-forming organisms, or gram-negative psychrotrophic spoilage organisms.
Unfortunately, if biofilms are not detected early enough, the result may be sporadic but persistent contamination that will decrease shelf life, introduce pathogens to finished products, or cause economic harm. If shelf life has already begun to fail due to higher-than-normal spoilage organism contamination, the possibility of biofilm formation must be considered, and strong measures must be taken to eliminate it. Aggressive cleaning procedures may be required to eliminate an established biofilm. Methods for evaluating and monitoring the cleaning results of a dairy plant or farm milk production operation should be built into a Hazard Analysis and Critical Control Point (HACCP) program and should include programs for evaluating processing areas for biofilm sanitation.
Hygienic Sampling is Key to Maintaining Good Manufacturing Practices
Biofilm formation represents a profound challenge to the dairy industry where they act as the principal reservoir of microbial contamination. This persistent threat of contamination by spoilage and pathogenic organisms thriving in biofilms has the potential to negatively impact product quality and safety that may lead to economic loss and damage brand reputation. Measures to control the introduction of contaminating microbes into dairy products and safeguarding against the development of biofilms on production or processing equipment are critical to protecting the food supply. Vigilance is needed to detect and isolate biofilms in dairy production and processing environments and find ways to eliminate them once formed. It is emphasized that good manufacturing practices, including good hygienic sampling practices and HACCP quality management systems implemented both at the farm and processing plant levels are vital to protect the quality and safety of the dairy supply. Management systems are vital to protect the quality and safety of the dairy supply.
Visit our website to download our Biofilms: A Persistent Challenge to the Dairy Industry to take a deeper dive into the role of biofilms in milk production and processing.
Additional resources:
Blogs:
Prevalence of Cold-tolerant Spore-forming Bacteria in the Milk Supply
Low-Spore Milk Production—The Mantra and its Benefits
Psychrotrophic Bacteria in Pasteurized Milk—Spoilage, Testing, and Line Sampling
Webinars:
Monitoring Microbial Contamination
2021 European Dairy Quality Conference (EDQC) Webinar
Training Materials:
QualliTru Applications and Product Guide
Why Process Monitoring & Aseptic Sampling Information Sheet
Best Practices for Using Your QualiTru Septum
Printable Site Application Schematics
Implementing Hygienic Sampling Practices is easy with QualiTru’s aseptic and representative sampling system.

Have questions about aseptic and representative sampling? Ask Our Experts




